Spontaneous coronary artery dissection (SCAD) is an increasingly recognized cause of acute coronary syndrome, especially among young to middle-aged women with few traditional cardiovascular risk factors and low pretest probability for atherosclerotic coronary artery disease. Diagnosis by invasive coronary angiography is the gold standard and conservative therapy is generally recommended, with percutaneous or surgical revascularization being reserved for cases of clinical instability, high-risk anatomy or as bailout. Unlike atherothrombotic coronary artery disease, strong evidence on optimal medical therapy is scarce, posing unique challenges in cases of pregnancy-associated SCAD. The follow-up strategy is also of major importance, as recurrent SCAD is not infrequent, lifestyle changes and pharmacological therapy should be planned for the long term, and SCAD-associated conditions need to be addressed. This review aims to provide a practical management approach to SCAD patients for both clinical and interventional cardiologists.
A dissecção coronária espontânea é uma causa cada vez mais reconhecida de síndrome coronária aguda, especialmente entre mulheres jovens ou de meia-idade com poucos fatores de risco cardiovascular tradicionais e baixa probabilidade pré-teste de doença coronária aterosclerótica. O diagnóstico por coronariografia invasiva é o gold standard e a terapêutica conservadora é geralmente recomendada, optando pela revascularização percutânea ou cirúrgica em casos de instabilidade clínica, anatomia de alto risco ou como bailout. Ao contrário da doença coronária aterotrombótica, não existe evidência científica robusta sobre a terapêutica médica mais adequada nestes casos, representando ainda desafios particulares em casos de dissecção coronária espontânea associada à gravidez. A estratégia de seguimento é também fundamental, dado que a recorrência de dissecção coronária espontânea não é rara, as alterações de estilo de vida e terapêutica farmacológica devem ser planeados em longo prazo e devem ser investigadas as condições subjacentes associadas à dissecção coronária espontânea. Esta revisão pretende oferecer um guia de abordagem prática ao doente com dissecção coronária espontânea, do ponto de vista do cardiologista clínico e do cardiologista de intervenção.
Spontaneous coronary artery dissection (SCAD) refers to the non-traumatic, non-iatrogenic development of a false lumen within the coronary artery wall, unrelated to the presence of atherosclerotic disease or primary aortic dissection.1 The dissection may originate from an intimal tear leading to dissection into the arterial wall or may result from spontaneous bleeding from ruptured vasa vasorum without intimal tear.2 Both mechanisms lead to compromised coronary blood flow and myocardial ischemia, either by expansion of an intramural hematoma (IMH) and external compression of the true lumen or thrombotic occlusion at intimal rupture sites.3 SCAD can be a cause of type 2 myocardial infarction (MI)4 and should be considered as a differential diagnosis of MINOCA (myocardial infarction with non-obstructive coronary arteries).5
While initially thought to be a very rare, pregnancy-associated condition, SCAD has been increasingly recognized as a cause of acute coronary syndrome (ACS), due to widespread use of high-sensitivity troponin assays for assessment of chest pain in the emergency department, routine coronary angiography in ACS and greater awareness of this disorder, prompting cardiologists to consider SCAD more often. According to contemporary registries, it accounts for 1–4% of all ACS,1,6,7 with pregnancy-associated SCAD representing less than 15% of cases.8
The patientThe majority of patients are women (>90% in most series),3 predominantly young to middle-aged (44–53 years old)7 at the time of diagnosis, although patients across the entire age spectrum have been reported. Although SCAD patients have lower rates of traditional cardiovascular risk factors than those with atherosclerotic MI, the prevalence of hypertension (27–32%), and hyperlipidemia (20–35%)9,10 is similar to that of unselected age- and gender-matched populations.
SCAD seems to result from the combination of a precipitating factor superimposed on an underlying arteriopathy that weakens the arterial wall (Table 1). Inciting triggers can be split into emotional and physical, most commonly intense emotional stress, strenuous exercise, labor and delivery, intense Valsalva-like activities, and use of recreational drugs or hormonal therapy.11 Many predisposing arteriopathies have been associated with SCAD, and extracoronary vascular abnormalities (EVAs) can be found in up to 70% if systematic screening is undertaken.11,12 Fibromuscular dysplasia (FMD), a non-inflammatory, non-atherosclerotic idiopathic arteriopathy with a predilection for medium-sized arteries, accounts for up to 50% of concomitant SCAD-associated vascular conditions,7 usually involving the carotid, renal and iliac arteries. Other connective tissue disorders including Marfan, Loeys-Dietz and Ehlers-Danlos type 4 syndromes, as well as systemic inflammatory conditions like systemic lupus erythematosus, inflammatory bowel disease and vasculitis-associated diseases, represent less than 5% of associated conditions.11,12
Predisposing conditions and precipitating triggers for spontaneous coronary artery dissection.3,11
| Predisposing conditions |
| Fibromuscular dysplasia |
| Connective tissue disorders (Marfan, Loeys-Dietz and Ehlers-Danlos type 4 syndromes, cystic medial necrosis, alpha-1 antitrypsin deficiency, polycystic kidney disease) |
| Systemic inflammatory disease (systemic lupus erythematosus, inflammatory bowel disease, celiac disease, vasculitis-associated diseases, sarcoidosis, rheumatoid arthritis) |
| Pregnancy-related (gestation and postpartum) |
| Hormonal therapy (oral contraceptives, infertility treatments, post-menopausal therapy) |
| Idiopathic |
| Precipitating triggers |
| Intense emotional stress |
| Intense physical stress (strenuous exercise, labor and delivery, Valsalva-like activities) |
| Recreational drugs (cocaine, amphetamines) |
Pregnancy and hormonal therapy are particularly important predisposing circumstances, given the predilection of SCAD for female patients and its association with pregnancy. Female sex hormones play a role, yet to be determined, in the pathophysiology of SCAD, probably related to hormonal influences on vascular connective tissue and microvasculature regulation.1 SCAD is the leading cause of MI among pregnant women,7,13,14 with most events occurring during the third trimester or first month post-partum.13 The few available studies suggest a more severe clinical course for this subset of patients, owing to more frequent involvement of the left main (LM) or proximal coronary segments, more extensive myocardial necrosis, and lower resulting ejection fraction.13 Patients with other female hormone-related conditions, ranging from infertility treatment to oral contraceptives and postmenopausal therapy,6 have a clinical course similar to other SCAD patients.
The diagnosisACS is the typical clinical presentation of SCAD, with a few patients coming to medical attention for ventricular arrhythmia (<10%), cardiogenic shock (<3%) or sudden death (<1%).3,11,15,16 The chief complaint (>95%) is pain, which has been attributed not only to myocardial ischemia and infarction, but also to vessel dissection per se.1 ST-elevation MI (STEMI) presentation ranges from 25% to 70% in published series,17 with the remainder being non-ST-elevation MI (NSTEMI), both involving elevated biomarkers and electrocardiographic ischemic changes or evidence of wall motion abnormalities.
While low pretest probability for atherosclerotic coronary disease might suggest a non-coronary diagnosis and lead an invasive approach to be postponed (mainly for patients presenting with NSTEMI), invasive coronary angiography is the gold standard for SCAD diagnosis1,6 and should follow recommended timeframes after ACS has been diagnosed.18,19 The interventional cardiologist should be aware of the angiographic patterns of SCAD to enable correct identification of SCAD and its types according to the Saw classification (Table 2).20 Type 2 SCAD (long, diffuse stenosis, usually >20 mm in length) is the most common type (70%), while the classical double-lumen arterial wall contrast staining appearance of type 1 corresponds to 25% of identified lesions. Type 3 (abrupt lumen narrowing with distal vessel size recovering, creating a focal lesion <20 mm in length) accounts for less than 5% of cases, and is the most difficult pattern to differentiate from atherosclerotic disease.20 Other common features are the involvement of the left anterior descending (LAD) artery (50–70%), the propensity for mid-to-distal coronary segments, and the presence of multivessel SCAD in 15–20%.21–25
Angiographic classification of spontaneous coronary artery dissection by Saw.20
| Type 1: contrast dye staining of arterial wall with multiple radiolucent lumen | 25% |
| Type 2: diffuse, smooth, long stenosis (>20 mm) | |
| Type 2A: stenosis bordered by normal proximal and distal arterial segments | 70% |
| Type 2B: stenosis extending to the apical tip of the artery | |
| Type 3: Focal or tubular stenosis (<20 mm) mimicking atherosclerosis | 5% |
When angiographic findings are ambiguous, intracoronary nitroglycerin (to relieve vasospasm) and intracoronary imaging are recommended to clarify the diagnosis.20 While intravascular ultrasound (IVUS) enables better wall vessel penetration to depict the entire depth of the IMH, optical coherence tomography (OCT) has superior spatial resolution for visualizing intimal tears, false lumens and IMH. Both techniques are recommended during the index coronary angiography if diagnosis in uncertain, with OCT preferred if intervention is pursued.3 Operators’ concern about additional coronary manipulation in the presence of arterial fragility may cause the use of intracoronary imaging to be reserved for ambiguous lesions (angiographic type 3 and some type 2), as well as for guiding and optimizing percutaneous coronary intervention (PCI) results.26 Despite the fear of iatrogenic injury, the distinct and contrasting patient management strategies between atherosclerotic and SCAD-associated ACS mean that correct diagnosis is key for deciding on ad hoc PCI versus a conservative approach and for planning appropriate medical treatment and follow-up.
Computed tomography (CT) coronary angiography, while safer than invasive angiography, lacks temporal and spatial resolution for adequate identification of most dissections, as the unique features of artery narrowing and IMH can be challenging to recognize or are unidentifiable in small vessels.6 Also, since the pretest probability for coronary disease in SCAD patients is often low, even proximal SCAD can be missed if not considered beforehand by the cardiovascular imaging interpreter.27 Echocardiography and cardiac magnetic resonance (CMR) play a role in the assessment of left ventricular (LV) function and in the differential diagnosis of cases labeled as MINOCA. However, neither should delay or replace prompt invasive coronary imaging.
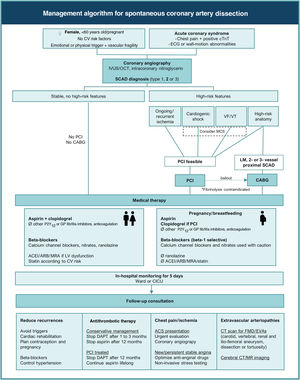
Conservative and interventional approachesThe recommendations from international societies for the approach and management of SCAD1,6 are summarized in Figure 1. Although the level of evidence is low, mostly based on observational data and expert opinion, these recommendations propose a uniform standard of care for SCAD patients, differentiating it from atherosclerotic ACS and pointing out management strategies associated with worse outcomes.
Management algorithm for spontaneous coronary artery dissection. Ø: not to be used; ACEI: angiotensin converting enzyme inhibitor; ACS: acute coronary syndrome; ARB: angiotensin receptor blocker; CABG: coronary artery bypass grafting; CICU: coronary intensive care unit; CT: computed tomography; cTnT: cardiac troponin; CV: cardiovascular; DAPT: dual antiplatelet therapy; ECG: electrocardiographic; EVAs: extracoronary vascular abnormalities; FMD: fibromuscular dysplasia; GP: glycoprotein; IVUS: intracoronary ultrasound; LM: left main; LV: left ventricular; MCS: mechanical circulatory support; MR: magnetic resonance; MRA: mineralocorticoid receptor antagonist; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; SCAD: spontaneous coronary artery dissection; VF: ventricular fibrillation; VT: ventricular tachycardia.
Adopting a conservative approach with medical therapy and in-hospital monitoring appears to be safer than intervention in most SCAD presentations, due to the commonly benign natural history of conservatively treated SCAD, which shows progressive and complete healing of the involved vessel within 30 days,11,22 and also the higher risk for complications and suboptimal results of PCI in this patient subset.15,28 A non-invasive therapeutic approach should thus be preferred when the patient is stable, even if symptomatic, at presentation.
After the diagnosis is made, aggressive antithrombotic strategies that could lead to propagation of the IMH should be avoided. According to this rationale, use of glycoprotein (GP) IIb/IIIa inhibitors is not advised, and anticoagulation should be immediately stopped after coronary angiography.1,6 Most experts recommend clopidogrel on top of aspirin, aiming at antagonizing the prothrombotic activity created by intimal fenestrations or flaps, and to avoid thrombosis of the true lumen induced by the expanding IMH and/or false lumen. The use of other P2Y12 inhibitors has been little reported, and their antiplatelet potency may be unnecessary and even deleterious for the above-mentioned reasons.
Other than antithrombotic agents, most guideline-directed pharmacological therapies for atherosclerotic ACS have an uncertain role in SCAD-associated ACS. Beta-blockers are the mainstay of therapy, by reducing contractility and coronary shear stress, in parallel with data that support their use in aortic dissection.29 Other antianginal drugs (such as calcium channel blockers, nitrates, ranolazine) are also valuable for symptom control.
Angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs) and mineralocorticoid receptor antagonists (MRAs) should be considered when LV dysfunction ensues.1,6 Statins are not mandatory and should be prescribed according to the estimated cardiovascular risk as for primary prevention patients,1,6,7 considering that SCAD events are not atherosclerosis-driven and do not amount to established atherosclerotic cardiovascular disease (ASCVD). However, patients with intracoronary imaging evidence of concomitant subclinical atherosclerosis not related to the event should be considered for statin therapy. Diabetic patients and others with documented ASCVD elsewhere should be managed accordingly.
Percutaneous coronary interventionPCI in SCAD patients is usually performed for high-risk presentations, including hemodynamic or electrical instability due to severe coronary flow limitation or high-risk anatomies (LM or multivessel proximal dissections).1,6 For STEMI patients, fibrinolysis is considered contraindicated, as IMH and dissection could extend even further after lytic therapy.1,6,17 During the index coronary angiography, isolated IMH without intimal tear (types 2 and 3) and more extensive and proximal dissections have been associated with a higher risk of early clinically important extension of SCAD23,30 if managed conservatively. When ongoing or recurrent signs of ischemia persist despite initial medical management, coronary angiography for reassessment of the dissection is advisable and PCI should be considered.
Overall, PCI for SCAD is associated with worse technical results (success rates from 47% to 91%3,28) and higher rates of complications (about 40–50%11,15,23) compared to PCI for other indications. Complications include iatrogenic coronary artery dissection (during diagnostic angiography or ad-hoc PCI31), dissection propagation with worsening TIMI flow, wiring and stenting of the false lumen, additional unplanned stents (including LM stenting), and emergency coronary artery bypass grafting (CABG).17
Choice of access should take into consideration the high prevalence of underlying vascular abnormalities when choosing the femoral route, together with the higher proportion of iatrogenic coronary artery dissection unrelated to the presenting SCAD segment when using radial access reported in some series.4,31 Radial access with dedicated catheters should avoid aggressive manipulation, given the high prevalence of concomitant subclavian and brachiocephalic tortuosity and take-over, as well as the risk associated with noncoaxial engagement in the coronary ostium and deep catheter engagement.
Compared with the approach in atherosclerotic disease, the focus of intervention in SCAD should be less on restoring normal coronary architecture and more on the minimal measures required to restore TIMI flow grade 3. Technical considerations include meticulous catheterization techniques, avoiding deep catheter engagement, noncoaxial positioning of the catheter tip, catheter dampening, and high-pressure contrast injections.31 Although it requires additional coronary instrumentation, intracoronary imaging should be used by default when PCI is performed. Both OCT and IVUS enable diagnosis and characterization of SCAD (differentiation between true and false lumens, identification of the proximal entry flap, extent of the dissection/hematoma, other vessel dissections) and aid in decision-making in the interventional strategy (target segment[s], balloon angioplasty versus stenting, and stent sizing) and guidance for wiring and stent placement.
Some experts advocate balloon angioplasty alone using undersized cutting balloons, aiming at fenestration of the false lumen and decompression of the IMH, thereby preventing propagation of the dissection.32 When stents are implanted, stent length should cover an additional 5–10 mm at the proximal and distal edges of the dissection, and diameter sizing should take into consideration the natural history of vessel healing in the short term, anticipating stent malapposition if undersized stents are used.17,32 No differences in PCI techniques are adopted according to the different angiographic patterns. Other technical tips from expert opinion17,32 are summarized in Table 3.
Percutaneous coronary intervention strategies.
| Femoral access preferred (?) |
| Meticulous guide catheter manipulation, avoiding deep catheter engagement, noncoaxial catheter intubation and forceful contrast injections |
| OCT/IVUS guidance to ensure wire position in true lumen and optimize stent apposition |
| Start with a floppy wire, then escalate to a hydrophilic wire or stiff wire if needed |
| Direct stenting without pre- or post-dilatation to reduce risk of hematoma extension |
| Possible careful use of cutting balloon (to fenestrate and decompress IMH) |
| Stent length covering 5–10 mm of proximal and distal edges of IMH |
| Multi-stent approach for long lesions (type 2), stenting proximal and distal edges first before placing a long mid-segment stent to prevent hematoma extension |
| Consider self-expanding stents (adapting to vessel healing and hematoma reabsorption) |
| Consider bioabsorbable stents (temporary scaffold to avoid long-term malapposition) |
| Consider follow-up OCT to assess for malapposed/uncovered struts before stopping dual antiplatelet therapy |
IMH: intramural hematoma; IVUS: intracoronary ultrasound; OCT: optical coherence tomography.
As for atherosclerotic coronary disease, CABG can be an option when large areas of myocardium are in jeopardy. Although SCAD classically involves mid-to-distal coronary segments, surgery should be considered as the first therapeutic approach when LM and/or two- or three-vessel proximal SCAD are diagnosed, or as bailout after failed or complicated PCI.1,6
Technical difficulties should be anticipated, particularly in correctly identifying the true lumen, avoiding previously implanted stents, and suturing the fragile, dissected coronary tissues; however, short-term outcomes seem to be favorable.15 Since there is no evidence in favor of arterial rather than venous grafts, the use of venous conduits seems a reasonable choice, even though higher rates of graft failure are reported in the long term,15 as coronary healing leads to competitive flow and favors graft occlusion.
Cardiogenic shock and mechanical circulatory supportWhen cardiogenic shock complicates SCAD, mechanical circulatory support should be considered as for non-SCAD ACS patients, according to international guidelines and consensus statements.18,19,33 Although there have been a few case reports suggesting that intra-aortic balloon pumps and extracorporeal membrane oxygenation are safe to use,6 caution is advised given the need for large-bore arterial access and the high incidence of concomitant arteriopathies in SCAD patients, including iliofemoral aneurysms, dissections and tortuosity.
Pregnancy-associated spontaneous coronary artery dissectionPregnancy-associated SCAD accounts for less than 10% of SCAD diagnoses, but SCAD represents about 50% of all acute coronary syndromes in pregnant women.13,14 Compared to other SCAD patients, LM and proximal vessel involvement are more common, and a more severe clinical course and worse outcomes have been reported. A high level of suspicion should be present, and the diagnostic approach should include coronary angiography, as the substantial maternal morbidity rates outweigh the risk of fetal radiation exposure when proper shielding measures are taken.7
As for other SCAD patients, a conservative approach should be the default, and PCI or CABG should only be considered in the previously mentioned settings. Drug safety during pregnancy and breastfeeding is also a major concern, and international guidelines should be followed.14 Positive safety data on low-dose aspirin and beta-blockers (preferably beta-1-selective drugs, as they are less likely to affect uterine contraction and fetal growth) make them the mainstay of therapy in SCAD. Clopidogrel should be confined to PCI-treated patients and for the shortest duration possible. Breastfeeding is not recommended in mothers taking antiplatelet agents other than low-dose aspirin. Also, there are no safety data available for other P2Y12 or GP IIb/IIIa inhibitors. Low molecular weight or unfractionated heparin can be used until coronary angiography is performed (preferably the latter, if prompt delivery is warranted); however, anticoagulation may be omitted if there is a high degree of suspicion for SCAD. Verapamil, diltiazem and nitrates can be used with caution as antianginal alternatives.34 Other commonly used drugs after ACS, including ACEIs, ARBs, MRAs and statins, are contraindicated during pregnancy and breastfeeding.
Hospital admissionAdmission to intensive care or to a ward with cardiac telemetry should be chosen according to clinical status. Postponing discharge until day 5–7 is usually recommended,3,15 as propagation of IMH and additional vessel dissection most often occur in the first few days in conservatively treated patients.30 This strategy allows for management of chest pain and recurrent ischemia, optimization of antianginal drugs and non-invasive ischemia testing as needed.
If the patient is stable and symptom-free, repeat coronary angiography to confirm healing is unnecessary, as angiographic healing is time-dependent, and 95% of conservatively managed patients show spontaneous recovery at 30 days post-SCAD.22 If chest pain recurs, the decision to perform early repeat coronary angiography should consider not only symptoms but also an electrocardiogram and measurement of biomarkers as for the initial ACS diagnosis. Pain may arise not from myocardial ischemia but from vessel injury and healing, and further coronary manipulation could result in additional iatrogenic vessel damage.1 SCAD extension may still be amenable to PCI or CABG; on the other hand, if repeat coronary angiography shows angiographic healing, defined as improvement of stenosis severity from the index event plus residual stenosis <50% plus TIMI flow grade 3,22 further investigation for alternative causes of chest pain should be performed.
LV function should be assessed by echocardiography, while CMR can play a role in the differential diagnosis if there is uncertainty after the index coronary angiography. The overlapping characteristics of SCAD and Takotsubo cardiomyopathy should be borne in mind, as both predominantly affect women, can be related to an emotional trigger, and may present with apical regional wall motion abnormalities,35 given the frequent LAD involvement in SCAD. A late gadolinium enhancement pattern suggestive of infarction in a coronary territory might help to differentiate the two, but a normal CMR does not reliably exclude SCAD.7
Standard prescription at discharge should include aspirin and a beta-blocker, adding a second antiplatelet agent for at least one month in conservatively managed patients and for 12 months in PCI-treated patients. ACEI, ARB, MRA and statin prescription should be tailored to LV function, cardiovascular risk and pregnancy status.
Follow-up consultationsFollow-up consultations should aim at preventing recurrence, planning medication discontinuation, managing chest pain and investigating SCAD-associated conditions.
Recurrent SCAD (defined as de novo spontaneous dissection with new recurrent ACS, which does not involve extension of dissection of the original SCAD lesion21) has been reported following the index SCAD event in up to 12–27% of cases (depending on follow-up duration),3,7 and accounts for the majority of recurrent MI events in this population. While emotional and physical triggers are commonly identified during the index admission, preventing both type of stimuli could prove unsuccessful and even deleterious for mental and physical health. Referral for cardiac rehabilitation programs is safe and recommended,6 while extreme endurance training, carrying heavy objects or efforts that require prolonged Valsalva maneuvers should be avoided.7
Regarding hormonal precipitating factors, recommendations should weigh the risks associated with pregnancy against those of any form of contraception. Women are often advised to avoid pregnancy after SCAD, but the few reports of maternal and fetal outcomes suggest that the majority of patients who subsequently conceive experience uncomplicated pregnancies,7 especially if previously asymptomatic and without LV dysfunction. Recent studies on women with a history of SCAD, comparing those who do and do not experience pregnancy after the first SCAD event, show similar risk of recurrence during follow-up.36 If pregnancy is undesirable, highly effective forms of contraception are recommended, preferably avoiding systemic estrogen-containing methods, and opting for locally acting intrauterine devices.6,7
Apart from avoidance of triggers, two main therapeutic targets have been associated with lower recurrence: beta-blocker use and hypertension control, in theory by reducing coronary artery wall stress. As such, while the evidence comes from observational data and no randomized controlled trials are available to date to further support these therapeutic measures, both should be addressed during each outpatient visit. Antiplatelets and other agents have not been shown to reduce the risk of recurrence, and discontinuation should be tailored according to the index SCAD treatment. Unlike the extensively studied antithrombotic therapy for atherosclerotic ACS (which should be applied for PCI-treated SCAD patients), there is lack of consensus on the use and duration of aspirin alone or dual antiplatelet therapy for conservatively managed patients. A reasonable approach is to prescribe dual antiplatelet therapy for 1–3 months4,7,37 and then continue low-dose aspirin for a total of 3–12 months. Should the bleeding risk be of concern, aspirin alone or no antiplatelet therapy is not unreasonable7 if stents have not been implanted.
During follow-up the cumulative burden of major adverse cardiac events is substantial, amounting to 15–20% at 6–7 years,11,16 and reaching almost 50% at 10 years,24 despite significantly better survival in the SCAD group compared with matched ACS controls.24 New ACS presentation should prompt urgent assessment, and differential diagnosis should include SCAD progression, recurrent SCAD and stent-related complications.6,7 For new-onset or persistent stable angina, optimizing medical management using long-acting nitrates, calcium channel blockers and ranolazine is advised, coupled with stress imaging to guide the need for repeat coronary imaging. Invasive coronary angiography is preferred, although coronary CT has shown to be a valid follow-up technique, especially for proximal-to-mid vessel dissections and for those that were not stented.27,38
Given the prevalence of over 50% of EVAs found in SCAD patients when systematic screening is undertaken,11,12 a neck-to-pelvis imaging protocol is recommended,39 looking for arterial dilations, aneurysms, dissections, and tortuosity. Conventional angiography is the gold standard, but CT imaging (preferred to magnetic resonance)27 is a more feasible one-step approach, aiming at identifying the multifocal ‘string of beads’ appearance of FMD through assessment of the carotid, vertebral, renal, and ilio-femoral arteries. Since intracerebral aneurysms may be present in 7–11% of patients,7 some experts support additional cerebral screening with magnetic resonance or CT scan, especially when diagnostic criteria for FMD are present.12 The therapeutic implications of this routine screening and need for serial imaging are not well established, but given the prevalence of FMD, diagnosis might allow timely management of aneurysms or dissection in vital territories. Other than in patients diagnosed with genetically linked vasculopathies, genetic testing and familial screening are unnecessary, as no monogenic cause for SCAD has been identified and few familial cases have been reported.1,6
SCAD patients are diagnosed with a rare, sometimes life-threatening condition, for which scientific evidence is scarce compared to other areas in cardiology, making patient education and expectation management challenging. Currently, international registries, such as the ongoing EURObservational Research Programme SCAD registry, aim to prospectively and retrospectively assess large cohorts of SCAD patients, aiming to expand knowledge and improve care of this challenging clinical entity.
ConclusionSCAD is an increasingly recognized but incompletely understood cause of ACS in young and otherwise healthy patients. Favorable short-term outcomes following conservative therapy contrast with high recurrence rates and long-term morbidity, requiring close follow-up for cardiac events. Prospective studies targeting pathophysiologic pathways and the role of disease specific interventions are warranted to improve SCAD prognosis.
Conflicts of interestThe authors have no conflicts of interest to declare.