Although not routinely used, cardioneuroablation or modulation of the cardiac autonomic nervous system has been proposed as an alternative approach to treat young individuals with enhanced vagal tone and significant atrioventricular (AV) disturbances.
We report the case of a 42-year-old athlete with prolonged ventricular pauses associated with sinus bradycardia and paroxysmal episodes of AV block (maximum of 6.6 s) due to enhanced vagal tone who was admitted to our hospital for pacemaker implantation. Cardiac magnetic resonance and stress test were normal. Although he was asymptomatic, safety concerns regarding possible neurological damage and sudden cardiac death were raised, and he accordingly underwent electrophysiological study (EPS) and cardiac autonomic denervation. Mapping and ablation were anatomically guided and radiofrequency pulses were delivered at empirical sites of ganglionated plexi. Modulation of the parasympathetic system was confirmed through changes in heart rate and AV nodal conduction properties associated with a negative cardiac response to atropine administration.
After a follow-up of nine months, follow-up 24-hour Holter revealed an increase in mean heart rate and no AV disturbances, with rare non-significant ventricular pauses, suggesting that this technique may become a safe and efficient procedure in this group of patients.
Ainda que raramente utilizada, a cardioneuroablação ou modulação do sistema nervoso autónomo cardíaco tem vindo a ser proposta como abordagem alternativa ao tratamento de indivíduos jovens com tónus vagal aumentado e, consequentemente, perturbações significativas da condução auriculoventricular (AV).
É apresentado um caso de um homem, atleta de 42 anos, referenciado para implantação de pacemaker por bradicardia sinusal e bloqueio auriculoventricular (BAV) de predomínio noturno por provável hipertonia vagal. As pausas noturnas por bradicardia sinusal associada a BAV atingiam os 6,6 segundos. Exames de imagem e prova de esforço foram normais. O doente foi submetido a estudo eletrofisiológico (EP) e desenervação autonómica cardíaca. O mapeamento e a ablação foram guiados anatomicamente, tendo sido aplicada energia por radiofrequência, de forma empírica, em locais da aurícula esquerda e direita onde previamente tinha sido descrita a existência de plexos ganglionares. A modulação do sistema parassimpático foi confirmada através da variação da frequência cardíaca e das propriedades de condução nodal AV no final do procedimento e ainda através de resposta cardíaca negativa à administração de atropina.
No seguimento de nove meses, o Holter de 24 horas evidenciou aumento da frequência cardíaca média e ausência de perturbações significativos da condução AV, sugerindo que essa técnica poderá vir a constituir uma alternativa à implantação de pacemaker neste subgrupo de doentes.
Sinus rhythm and heart rate (HR) are regulated by the cardiac autonomic nervous system, which consists of both sympathetic and parasympathetic nervous systems.1,2 Unlike the sympathetic nervous system, the latter reduces cardiac automatism, excitability and conductivity. Paraympathetic ganglionated plexi (GP) are located outside the atrial wall, in the surrounding regions of the sinus and atrioventricular (AV) nodes in close proximity to the myocardium (and are thus amenable to ablation),1,2 and exert their action through short postganglionic neural terminations.
It is well known that an imbalance between the sympathetic and parasympathetic systems may be responsible for various clinical conditions that include symptomatic or asymptomatic sinus bradycardia, ventricular pauses or AV block, which can be transient or permanent, even in the absence of fibrodegenerative disease of the conducting system.3,4
In high-performance athletes, bradyarrhythmias may occur as a result of increased vagal tone and are mostly asymptomatic.5 Uncertainty regarding the need for specific therapy is raised in the presence of extremely long ventricular pauses or high-degree AV block.6–8
Affected individuals usually refuse to have a pacemaker implanted, as this involves several concerns such as lead and generator replacements during follow-up, higher risk of infection, and the possibility of developing ventricular dysfunction due to long-term ventricular pacing,9 which suggests that parasympathetic denervation could be a potential alternative therapeutic strategy for these patients in the future.3,4,10,11
Epicardial GP have been managed from the endocardial surface, targeted using different techniques of radiofrequency catheter ablation (RFCA).3,12–15
We report the case of a young high-performance athlete with frequent episodes of transient AV block and long ventricular pauses who underwent a cardioneuroablation procedure.
Case reportA 42-year-old man was referred to our hospital for implantation of a permanent pacemaker. A resting 12-lead screening electrocardiogram (ECG) had shown sinus bradycardia and a 24-hour Holter revealed long ventricular pauses in association with high-degree AV block.
He was clinically asymptomatic, had no previous clinical history and denied using any medication or other drugs. He ran 18 km five times per week and would participate in one marathon per month on average.
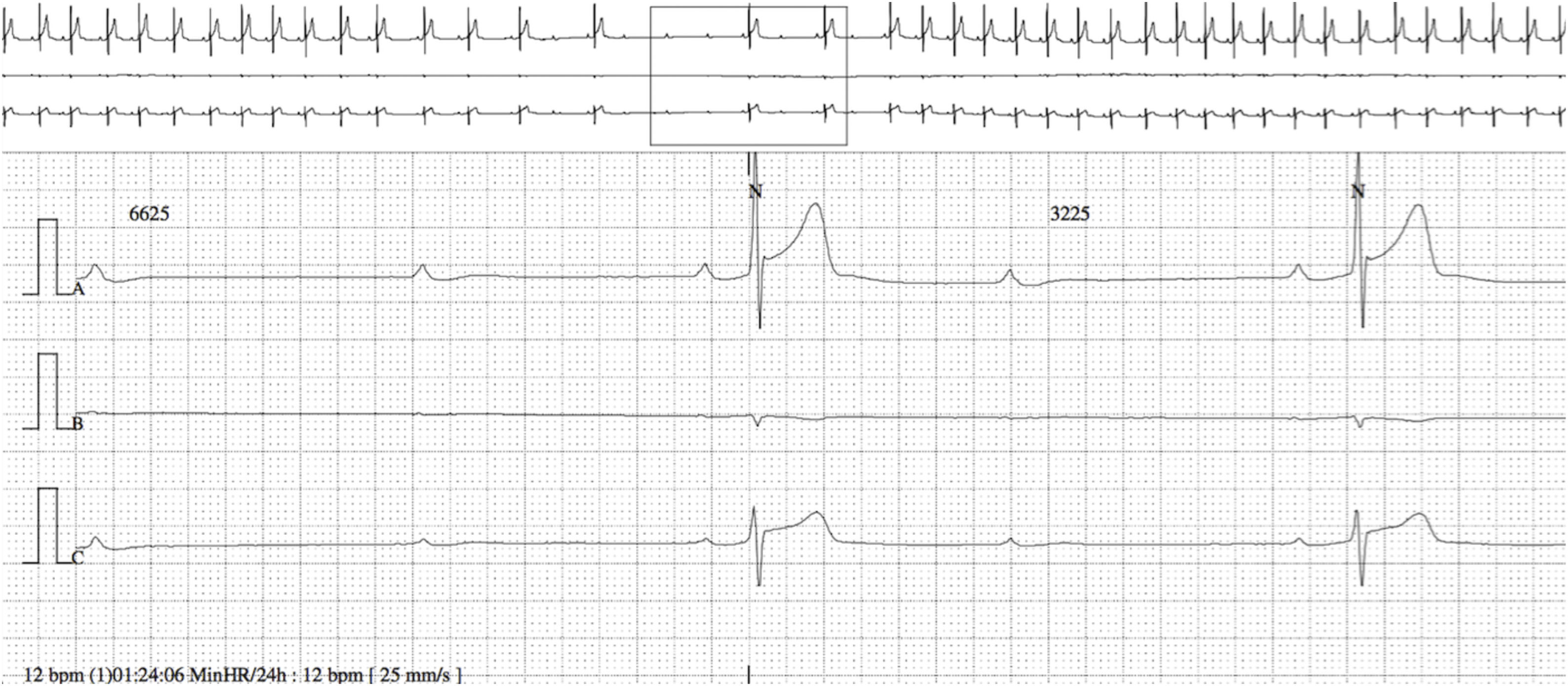
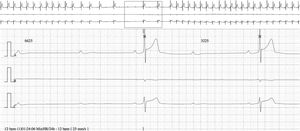
The baseline ECG showed sinus bradycardia with first-degree AV block, at an HR of 40 bpm (PR interval 250 ms, QRS interval 80 ms and QT interval 440 ms). There were no repolarization abnormalities. Maximum HR was 85 bpm and minimum was 12 bpm on 24-hour Holter; mean HR during the day was 45 bpm. Periods of first- and second-degree Mobitz type I AV block and episodes of nocturnal advanced AV block were documented, the longest ventricular pause being at 1:24 am (Figure 1), with a total duration of 6625 ms (within a total of 328 pauses). There were no premature ventricular or supraventricular contractions. Exercise stress testing showed a normal chronotropic response. Echocardiography and cardiac magnetic resonance imaging revealed no structural heart disease.
The patient was advised to discontinue vigorous physical activity. However, as he refused, an electrophysiological study (EPS) and cardiac vagal denervation were proposed and accepted as an alternative.
Under conscious sedation, the patient underwent EPS with 12-lead ECG and intracardiac electrograms displayed simultaneously on a multichannel recorder. Three multipolar catheters were placed in the coronary sinus, His bundle and left atrium through transseptal puncture from the right femoral vein. Heparin was infused and activated clotting time was maintained between 300 and 350 s. Basic electrophysiological assessment showed an initial HR of 45 bpm (RR interval 1333 ms), atrial-His (AH) interval of 200 ms, His-ventricular (HV) interval of 50 ms, Wenckebach cycle length of 1000 ms and a sinus node recovery time of 1779 ms (corrected 446 ms).
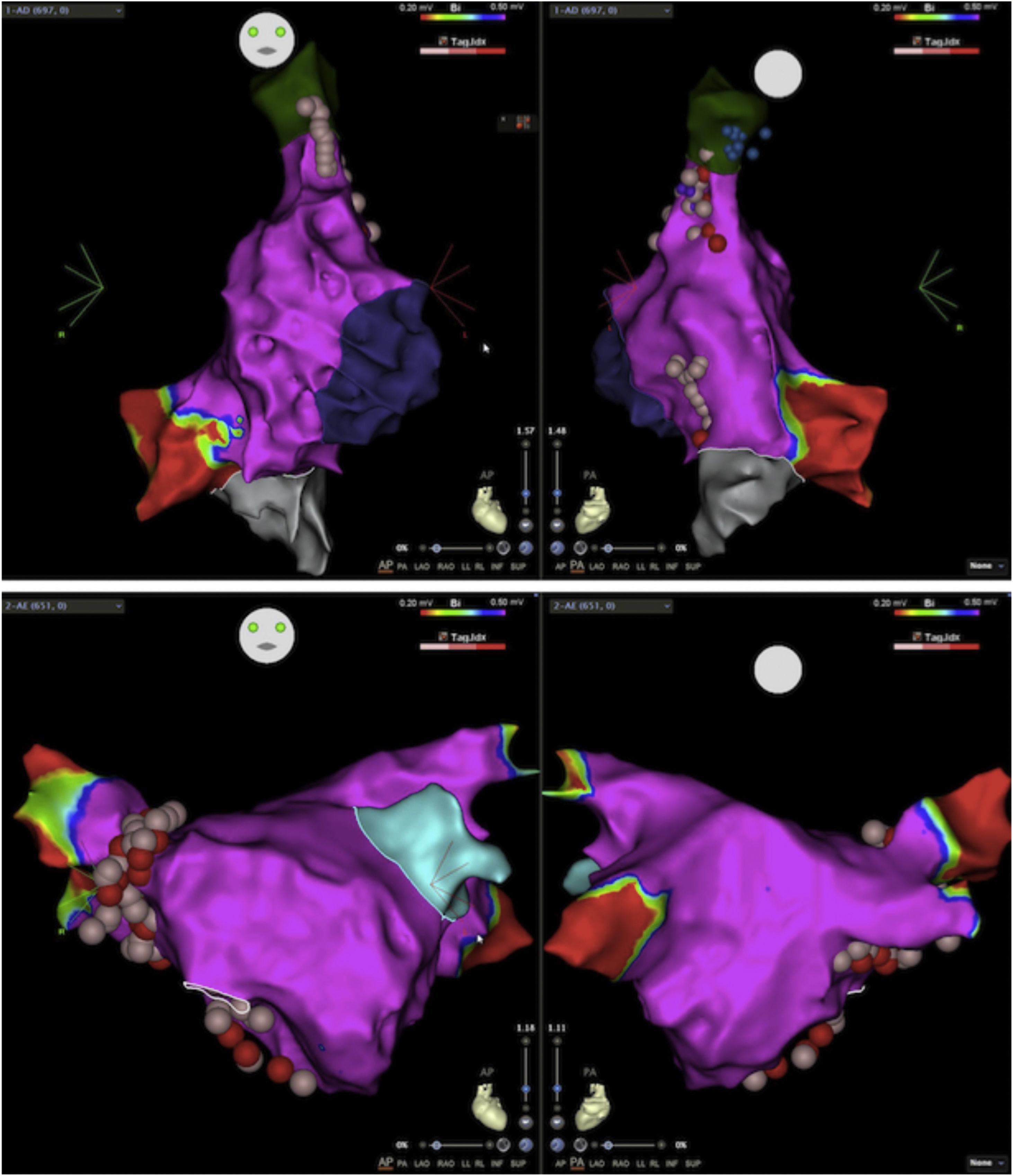
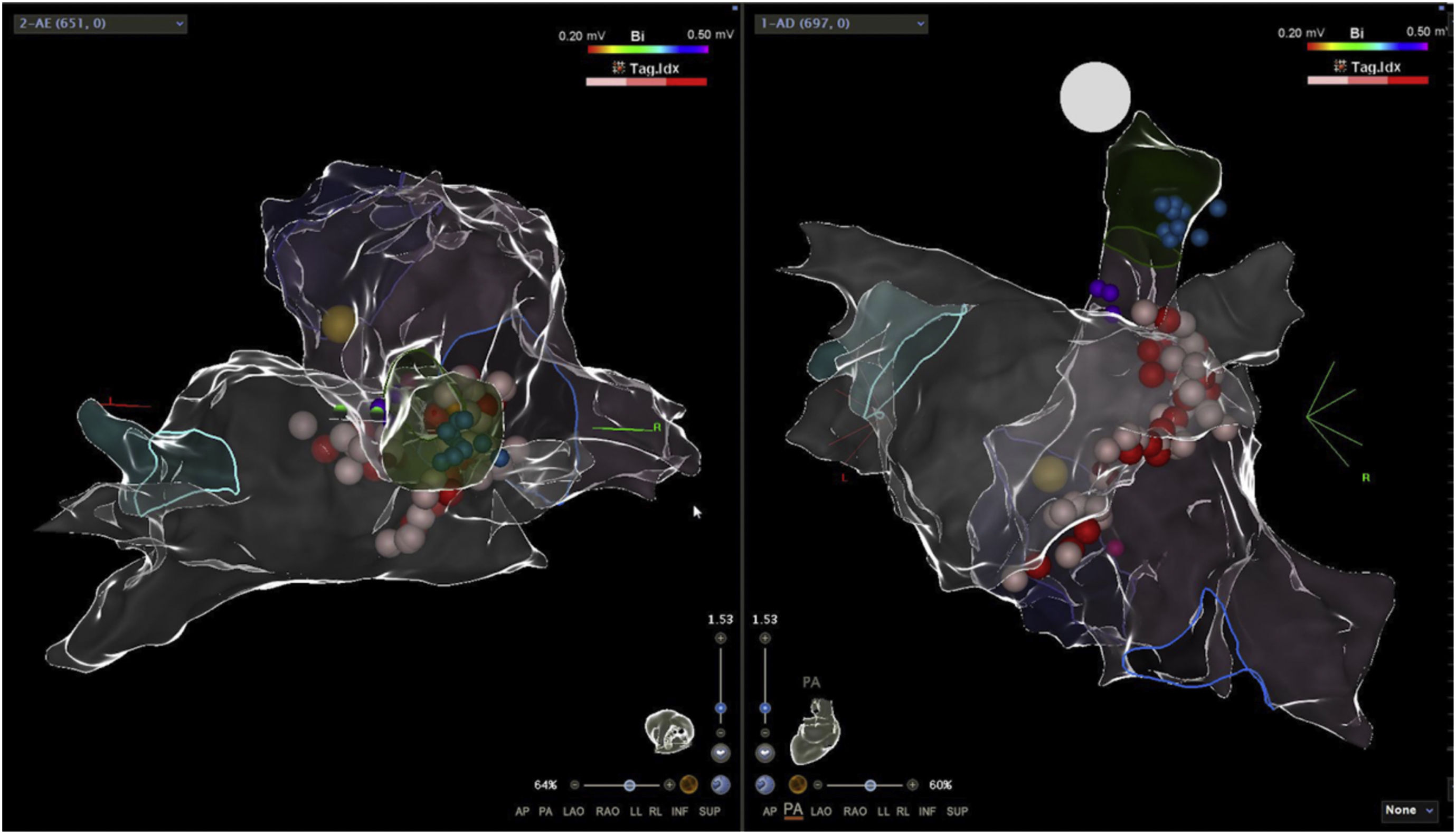
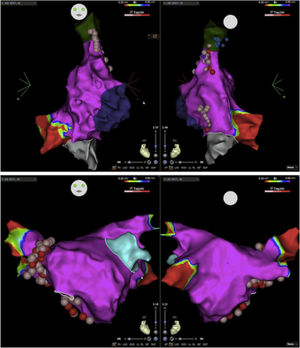
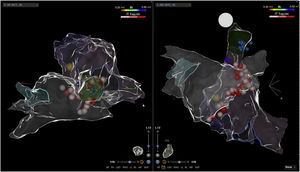
Electroanatomic mapping of the right and left atria was performed using the CARTO 3 system and a PentaRay catheter (Biosense Webster Inc., Diamond Bar, CA, USA). Unlike other reported cases that used spectral mapping to localize endocardial vagal innervations,3,10,16 in our case all targets were empirically identified by presumed anatomic location described in previous studies3,10–13,17: in the left atrium (LA), the inferior right GP (posterior aspect of the inferior right pulmonary vein) and the anterior right GP (anterior aspect of the right superior pulmonary vein); in the right atrium (RA), the posterior aspect of the interatrial septum, between the posterior wall and coronary sinus ostium and the septal aspect of the superior vena cava junction (Figures 2 and 3). Radiofrequency pulses (20–30 W, 50°C) were delivered with a 3.5 mm irrigated (12 ml/min) tip catheter (THERMOCOOL SMARTTOUCH SF; Biosense Webster Inc., Diamond Bar, CA, USA). Contact force ranged between 10 and 30 g.
Electroanatomic map with anteroposterior and posteroanterior views of the right atrium showing radiofrequency (RF) applications (pink and red dots) on the septal aspect of the superior vena cava and near the coronary sinus ostium (above); left atrium and RF applications on the anterior aspect of the right pulmonary veins (below).
Eventually, changes were observed in HR from 45 to 65 bpm and in Wenckebach cycle length from 1000 to 620 ms. The AH interval shortened to 146 ms and the HV interval did not change. Intravenous atropine bolus (0.04 mg/kg, up to a maximum of 3 mg in a single dose) did not increase HR.
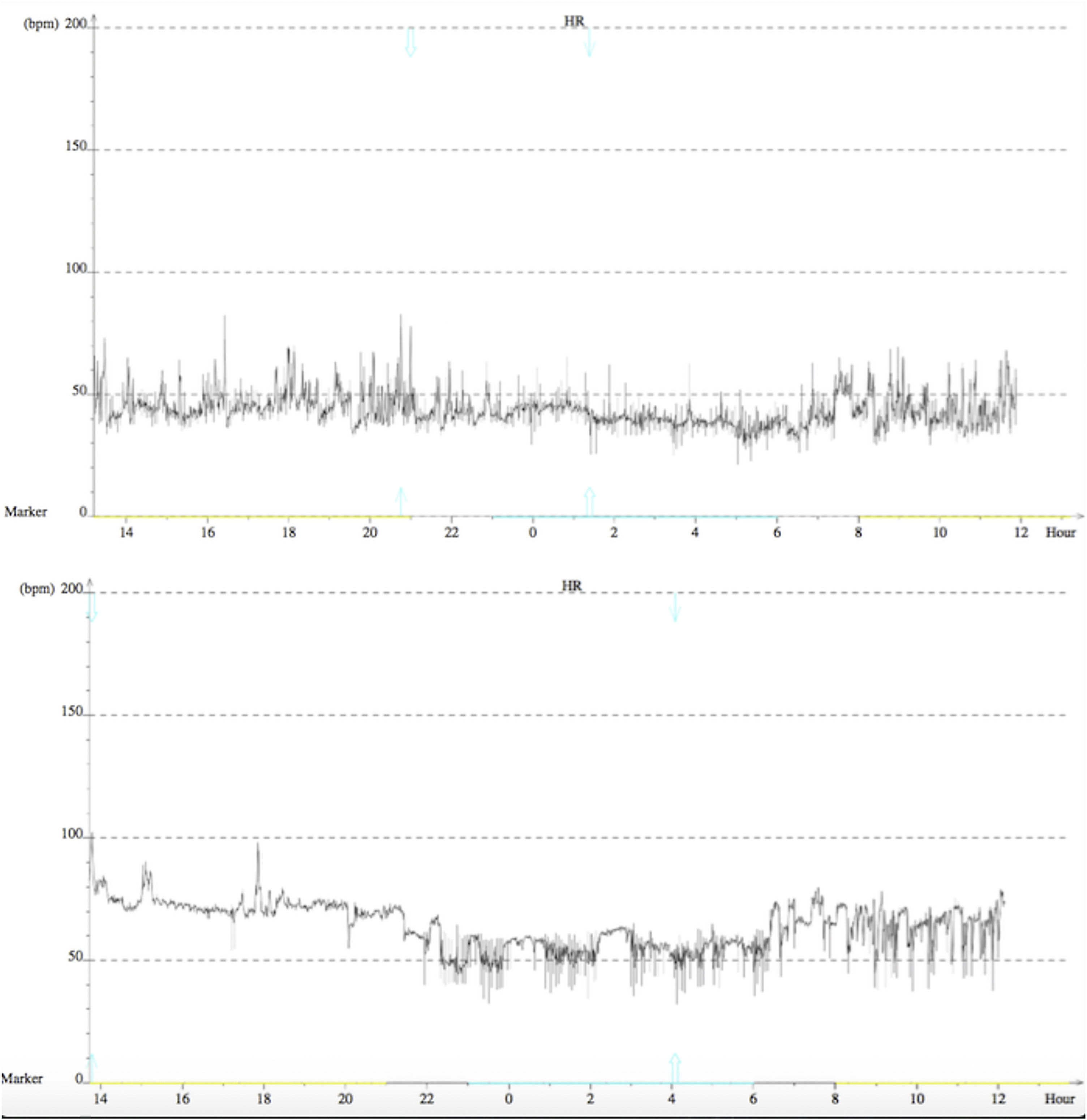
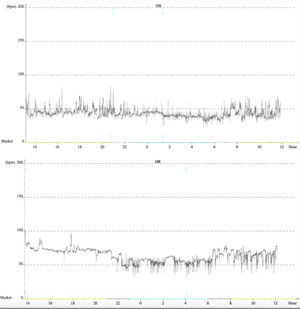
After three months, the patient remained asymptomatic and maintained his usual activities. A 24-hour Holter recording revealed improved results: maximum HR of 77 bpm, minimum of 46 bpm and mean of 59 bpm. The longest RR interval was 1365 ms and there was no evidence of AV block and no premature ventricular or supraventricular contractions. At nine months of follow-up, 24-hour Holter still showed sinus rhythm, with maximum HR of 103 bpm, minimum of 36 bpm and mean of 55 bpm (Figure 4). There was a nocturnal period of Mobitz type 1 second-degree AV block, but no other significant AV disturbances. No complications were observed during the procedure or in follow-up.
DiscussionIt has been known for years that the autonomic nervous system has a significant effect on cardiac electrophysiology and is implicated in the origin of various arrhythmias. It is thus an attractive target for their management. Initially, neural modulation emerged as a potential therapy in patients with long QT syndrome or catecholaminergic ventricular tachycardia who had ventricular tachycardia refractory to medical therapy that could be reduced by left stellate ganglion ablation.18 However, after the realization that vagal stimulation was proarrhythmic in the atria, by reducing the atrial effective refractory period, studies were carried out to assess the role of cardiac denervation in the treatment of vagally triggered atrial fibrillation. In this regard, Pachon et al., studying the spectrum of atrial potentials by fast Fourier transform, defined ganglionic plexi, ablation of which would reduce paroxysmal AF19 and gave rise to cardioneuroablation. A year later, using a different technique, Scanavacca et al. examined whether selective RFCA of atrial sites in which high-frequency stimulation induces vagal reflexes prevented paroxysmal AF12 and three years later, Katritsis et al. published similar endpoints using an anatomical approach.13
These developments led to the investigation of cardioneuroablation as a potential treatment in the management of bradyarrhythmias and as an alternative to pacemaker implantation, since it would avoid multiple lead/pacemaker replacements, risk of infection, and long-term nonphysiological pacing and associated secondary atrial enlargement and ventricular dysfunction.3,10,16,20–22 Since the first description by Pachon et al.,3 several other small studies and case reports have been published,14,20,21,23 especially in young patients with enhanced vagal tone and consequent neurally mediated syncope, intermittent high-degree AV block or sinus node dysfunction. In our case, the patient presented with ventricular pauses in association with sinus bradycardia and advanced AV block in the context of enhanced vagal tone and, although asymptomatic, he underwent the procedure to reduce the risk of neurological damage and sudden cardiac death due to bradycardia-dependent ventricular arrhythmias, as described in several studies.6–8 However, the inclusion criteria are still the subject of debate, and confirming the functional nature of the disorder, through normal chronotropic competence, normal SA nodal function and a Wenckebach point at suprahisian level, is crucial.10,15
Several mapping techniques to localize parasympathetic GP have been described.3,12,14,15,20,22 Unlike other studies that used high-frequency stimulation, spectral analysis or fractionated atrial electrograms to localize cardiac vagal innervation, our procedure was anatomically guided and restricted to the atrial septum, as previously described.10 RF pulses were therefore empirically delivered at the presumed anatomic location of the anterior right GP, which is known to provide most of the parasympathetic innervation for the sinus node, and of the inferior right GP, responsible for AV nodal innervation,2,22,24 on both sides of the atrial septum. The procedure was ended when an increase in HR, decreases in Wenckebach cycle length and AH interval and a negative response to atropine administration were achieved.
Finally, there is the question whether this technique is able to achieve prolonged and satisfactory results. Similarly to other case reports,11 at nine months of follow-up our patient remained asymptomatic and 24-hour Holter showed no significant AV disturbances, suggesting that these procedural targets could lead to appropriate vagal tone modification (Figure 4). Indeed, in theory, as the parasympathetic GP are located in the fatpad in close proximity to the myocardium, RFCA in their presumed anatomic location could provide permanent reduction of parasympathetic effects. The sympathetic and sensory systems would not be permanently affected since they only have postganglionic nerve terminations in this region, which can regenerate with time.1,2,23 However, during the procedure it is impossible to know whether permanent damage is done to the GP themselves or only to their short postganglionic nerve terminations. Accordingly, due to our short follow-up, we still cannot be sure if cardioneuroablation causes only a temporary effect while parasympathetic postganglionic axons are regenerating, or a permanent one.
LimitationsDespite the positive results, the present study refers to clinical data of only one case, with a relatively short follow-up time, and hence with unknown long-term results. Late vagal tone recovery after cardioneuroablation needs to be taken into consideration. For instance, although it might be possible to achieve broad vagal denervation by anatomically ablating overlapping GP areas in the atrial endocardium,10 the presence of postganglionic parasympathetic neurons and of higher innervation density along the atrial walls, which can only be identified by electrophysiological and/or spectral mapping, may contribute to incomplete denervation or even allow enough reinnervation for relapse.25,26
Considering the resulting minimum and maximum HR and the short nocturnal Wenckebach episode, the response in our patient appears to be only partial; however, it was sufficient to solve the clinical problem, avoiding pacemaker implantation.
Another important issue concerns the immediate endpoints used to define proper assessment of denervation. Although the RR interval, Wenckebach cycle length and nodal conduction properties combined with a negative response to atropine were taken into consideration, there was no previous atropine test with which to compare. The lack of atrial stimulation to assess the Wenckebach cycle during the procedure and after atropine infusion was a significant limitation. Also, atropine presents a long-lasting autonomic effect, promoting an immediate HR increase, and, in the event of additional ablation being required, it limits any further assessment. As an alternative, Pachon et al. suggested endovenous vagal stimulation using a bipolar electrophysiological catheter, which should be considered.25
Furthermore, it is important to mention that this technique requires transseptal puncture, and thus entails potential risks.
ConclusionWe describe a case of successful cardioneuroablation that, although limited by its short follow-up and the need for more long-term analysis, raises the hypothesis that modulation of the cardiac autonomic nervous system, aiming to modify parasympathetic tone through anatomically guided ablation, could become an alternative for young patients with enhanced vagal tone requiring device implantation. We believe this novel technique should be evaluated on a larger scale, with randomized controlled trials, to assess its safety and efficacy, so that it could potentially be included as a routine alternative treatment in the management of bradyarrhythmias.
Conflicts of interestThe authors have no conflicts of interest to declare.