Transcatheter aortic valve replacement (TAVR) has changed the treatment paradigm of severe aortic stenosis (AS). Nevertheless, in Portugal the penetration rate of TAVR is still very low and there is a paucity of data regarding its economic impact on the Portuguese healthcare system.
AimsTo perform an economic analysis of the present and future impact of TAVR in Portugal and to propose health policy recommendations for a new reimbursement model.
MethodsHospital data from a high-volume center were used as a sample to calculate the costs of TAVR in Portugal. Information regarding the national penetration rate was derived from the EAPCI Valve for Life initiative. To estimate the future demand for TAVR, three scenarios (S) were constructed: S1, TAVR penetration according to current guidelines; S2, including intermediate-risk patients; and S3, including low-risk patients aged over 75 years.
ResultsThe total cost of each TAVR procedure in Portugal was 22 134.50 euros for the self-expanding valve (SEV) and 23 321.50 euros for the balloon-expanding valves (BEV). Most of the cost was driven by the price of the valve (SEV 74.5% vs. BEV 81.5%). The current national economic impact is estimated at 12 500 000 euros per year. In S1, the expected penetration rate would be 189 procedures per million population; in S2 we estimated an increase of 28% to 241 procedures per million population and in S3 an increase of 107% to 391 procedures per million population. The total economic impact would increase to 43 770 586 euros in S1 and to 90 754 310 euros in S3.
ConclusionsTAVR is associated with a significant present and future economic impact on the Portuguese healthcare system. A new model of reimbursement in Portugal should be discussed and implemented.
A válvula aórtica percutânea (VAP) mudou o paradigma de tratamento da estenose aórtica grave. No entanto, em Portugal, a sua penetração é reduzida e dados acerca do impacto económico no Serviço Nacional de Saúde (SNS) são escassos.
ObjetivosAnalisar o impacto económico atual e futuro do implante de VAP em Portugal e propor um modelo de reembolso específico para as VAP.
MétodosOs custos fixos e variáveis do procedimento em Portugal foram calculados com base numa amostra de um centro de elevado volume de VAP. O impacto económico nacional foi calculado com base na penetração atual reportada na iniciativa EAPCI Valve for Life. As necessidades futuras da tecnologia foram estimadas em três cenários (S): S1 - penetração de acordo com as recomendações atuais; S2 - inclusão de doentes de risco intermédio; S3 - expansão aos doentes de baixo risco e idade > 75 anos.
ResultadosO custo total da VAP em Portugal foi estimado em 22.134,5€ com prótese autoexpansível (SEV) e 23.321,5€ com prótese expansível por balão (BEV). A maior parte do custo relacionou-se com o preço da prótese (SEV 74,5% versus BEV 81,5%). O impacto económico nacional estima-se em 12.500.000€/ano. No cenário S1, a taxa esperada de penetração deveria ser 189/milhão; em S2 perspetiva-se aumento de +28% para 241 procedimentos/milhão; em S3 estima-se aumento de +107% para 391 procedimentos/milhão. Assim, o impacto económico poderá aumentar significativamente de 43.770.586€ (S1) até 90.754.310€ (S3).
ConclusõesA VAP está associada a um impacto económico presente e futuro significativo no SNS. É essencial discutir e implantar um novo modelo específico de reembolso em Portugal.
In most European countries, healthcare spending has been rising at higher rates than gross domestic product, imposing increasing challenges for the sustainability of healthcare systems.1 New medical technologies have been a key driver of this increase in health spending.2,3 There is thus a need to assess the cost-effectiveness and economic impact of the adoption of new technologies before their dissemination in clinical practice. The wide variety of reimbursement models in different healthcare systems means that these economic assessments should be individualized and country-specific.4
Aortic stenosis (AS) is the most frequent type of valvular heart disease in Europe and North America,5,6 and its prevalence is expected to increase exponentially in the coming decades due to the aging of the population.7,8
In the last decade, transcatheter aortic valve replacement (TAVR) has emerged as a new technology to treat patients with severe AS who are considered inoperable or at high risk for surgery.9–12 New clinical trials have broadened the clinical indications for the procedure, demonstrating its clinical efficacy also in patients with intermediate13,14 and low risk15,16 as a replacement for traditional surgery. There is thus a growing replacement effect, with more patients now treated with TAVR than surgery in some countries, for example in Germany.17,18 This will impose significant challenges for the organization of hospitals and carries a high economic burden for healthcare systems, since the price of the transcatheter valve is 4-5 times higher than that of the valve used for surgical replacement.19 With projected estimates of up to 270000 TAVR candidates per year in Europe and North America, at a total estimated cost of $13.7 billion and $7.2 billion, respectively, the economic impact TAVR is set to be be enormous.20
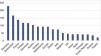
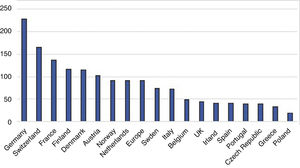
In Portugal, the technology was introduced in 2007 but penetration rates have been very low compared to other European countries (Figure 1),21 mainly because of the reimbursement model.22 Given the lack of economic data on TAVR in Portugal, it is important to determine its real-world costs, to assess its economic impact on local and national hospital budgets, and to predict future demand for the technology.
Penetration rates of transcatheter aortic valve replacement in different European Union countries per million population (adapted from EAPCI Valve for Life Initiative, 2016 data21).
The aim of this study was to perform an economic analysis of the present and future impact of the introduction of TAVR on the Portuguese healthcare system. More specifically, we aimed to calculate the current costs of a TAVR procedure in Portugal and to assess its economic impact at national level. Moreover, based on country-specific demographic and disease prevalence data, we estimated future demand for this technology according to different clinical scenarios.
MethodsEconomic impact at hospital and national levelTo calculate the real costs of TAVR in the Portuguese healthcare system we selected, as a sample, the information collected from a high-volume Portuguese TAVR center, the cardiology department of Centro Hospitalar de Vila Nova Gaia. Using official hospital information from 2017, we prospectively collected data about all the material used (quantity consumed and price per unit), costs of human resources and costs of hospital facilities. Costs were calculated separately for each type of valve.23
As detailed in Table 1, costs were subdivided into preprocedural, procedural and postprocedural costs.
Detailed costs (in euros) of the transcatheter aortic valve replacement procedure in a Portuguese tertiary hospital (Gaia Hospital Center).
| Self-expanding valve (Medtronic) | Balloon-expanding valve (Edwards) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cost/unit | n | Probability | Total | Cost/unit | n | Probability | Total | |
| Preprocedural costs | ||||||||
| Transesophageal echo | 277.8 | 1 | 277.8 | 277.8 | 1 | 277.8 | ||
| CT scan | 196.6 | 1 | 196.6 | 196.6 | 1 | 196.6 | ||
| Heart team assessment | 71.5 | 1 | 71.5 | 71.5 | 1 | 71.5 | ||
| Blood tests | 42.0 | 1 | 42.0 | 42.0 | 1 | 42.0 | ||
| Subtotal | 587.9 | Subtotal | 587.9 | |||||
| Fixed procedural costs | ||||||||
| a) Material | ||||||||
| Sheaths (6F/8F) | 14.5 | 1 | 14.5 | 14.5 | 1 | 14.5 | ||
| Sheaths for valve (12/14F) | 183.3 | 1 | 183.3 | -a | 0 | 0 | ||
| Sheaths – radial 6F (Terumo) | 38.5 | 1 | 38.5 | 38.5 | 1 | 38.5 | ||
| Guiding catheter R 250 | 14.8 | 1 | 14.8 | 14.8 | 1 | 14.8 | ||
| Guiding catheter J 250 | 14.8 | 1 | 14.8 | 14.8 | 1 | 14.8 | ||
| Guiding catheter (hydrophilic) 250 | 95.9 | 1 | 95.9 | 95.9 | 1 | 95.9 | ||
| Guiding catheter (stainless) 0.35 260 | 123.0 | 1 | 123.0 | 123.0 | 1 | 123.0 | ||
| Catheter (pigtail) | 14.8 | 2 | 29.5 | 14.8 | 2 | 29.5 | ||
| Catheter (AL1) | 14.8 | 1 | 14.8 | 14.8 | 1 | 14.8 | ||
| Temporary pacemaker | 92.3 | 1 | 92.3 | 92.3 | 1 | 92.3 | ||
| Valvuloplasty balloon | 1088.6 | 1 | 1088.6 | - a | 0 | 0 | ||
| Vascular closure (Proglide®) | 184.5 | 2 | 184.5 | 184.5 | 2 | 184.5 | ||
| Hemodynamic kit | 46.4 | 1 | 46.4 | 46.4 | 1 | 46.4 | ||
| Medication during procedure | 24.2 | 1 | 24.2 | 24.2 | 1 | 24.2 | ||
| Extra sterilized surgical gowns, drapes and suits | 1.6 | 2 | 3.2 | 1.6 | 2 | 3.2 | ||
| Contrast | 150.0 | 1 | 150.0 | 150.0 | 1 | 150.0 | ||
| Valve | 16 500.0 | 1 | 16 500.0 | 19 000.0 | 1 | 19 000.0 | ||
| Subtotal | 18 778.3 | Subtotal | 20 006.4 | |||||
| b) Human resources | ||||||||
| Anesthesiologist, ×1 (per hour) | 27.0 | 2 | 54.0 | 27.0 | 2 | 54.0 | ||
| Nurses/technicians, ×4 (per hour) | 10.5 | 8 | 84.0 | 10.5 | 8 | 84.0 | ||
| Cardiologists, ×2 (per hour) | 27.0 | 4 | 108.0 | 27.0 | 4 | 108.0 | ||
| Subtotal | 246.0 | Subtotal | 246.0 | |||||
| c) Hospital facilities | ||||||||
| Angiography room, per hour of use (2017 data) | 55.0 | 2 | 110.0 | 55.0 | 2 | 110 | ||
| Intensive cardiac care unit, per day (2017 data) | 418.0 | 3.5 | 1463.0 | 418.0 | 3.5 | 1463.0 | ||
| Hospital ward, per day (2017 data) | 136.0 | 4.5 | 612.0 | 136 | 4.5 | 612.0 | ||
| Subtotal | 2185.0 | Subtotal | 2185.0 | |||||
| Variable procedural costs (according to the risk of complications) | ||||||||
| Risk of pacemaker implantation | 1176.8 | 1 | 0.16 | 188.3 | 1 176.8 | 1 | 0.15 | 176.5 |
| Risk of vascular complication(includes sheaths, balloons, stents) | 1841.1 | 1 | 0.081 | 149.1 | 1 841.1 | 1 | 0.065 | 119.7 |
| Subtotal | 337.4 | Subtotal | 296.2 | |||||
| Total | 22 134.5 | Total | 23 321.5 | |||||
The costs of preprocedural exams (transesophageal echocardiography, computed tomography and blood tests) were obtained from the official table of costs of complementary diagnostic and therapeutic procedures of the Portuguese Ministry of Health for 2018.24 Before the procedure, the patient usually undergoes assessment by a multidisciplinary heart team that includes an interventional cardiologist, a cardiac imaging expert, an anesthesiologist and a cardiac surgeon, to assess their suitability for the procedure. The attributed cost was 71.5 euros of this assessment, which is the sum paid to the hospital, according to the Ministry of Health's 2018 official table of costs.
Procedural costsRegarding material costs, the price per unit was obtained individually for each item using 2017 official hospital consumption prices, after reductions or discounts. The medications used included heparin, propofol, droperidol, fentanyl, paracetamol, midazolam and cefazolin.
To calculate the costs of human resources, we considered the average salary of a Portuguese doctor (2746 euros/month), which including subsidies, bonuses and other extras, represents an annual cost of 48719 euros. Considering that the mean number of working hours is 151.7 per month, the cost per hour was estimated at 27 euros. The costs are the same for cardiologists or anesthesiologists. Two interventional cardiologists and one anesthesiologist are needed for a standard TAVR procedure. The procedure takes on average two hours, resulting in a total of six hours of medical work. The same method was used to calculate the price per hour of a nurse, which was 10.50 euros. At least four nurses are needed during the procedure, which corresponds to eight hours of nursing work.
Regarding the costs of hospital facilities, no detailed information was previously available about the cost per day of stay in the cardiac intensive care unit or on the cardiology ward. Therefore, this price was determined prospectively using hospital information from 2017. Information on the median time of hospitalization from January 2017 to January 2018 was used to calculate the median time of hospitalization after the procedure.
To estimate the costs per hour of use of the angiography room, we estimated that the total investment for an angiography room (including angiographic and other equipment and construction work) was 900000 euros, which was the total cost of such a room in this hospital acquired in 2017. Maintenance costs over the room's 10-year lifetime, 450000 euros in total, were added to this figure. Considering the number of hours of use (11hours per day in our center) on 250 days per year (to account for weekends, holidays, maintenance days and strikes, according to 2017 data), the estimated cost per hour of use was 55.0 euros.
The costs were subdivided into two types: fixed and variable costs (the latter depending on the risk and type of complications). To more accurately reflect the real impact of variable costs, we retrospectively collected information on the risk and types of complication in this hospital in the previous year (from January 2017 to January 2018), to take into account the TAVR learning curve.25 Because the risks and types of complication can differ according to the type of valve, a separate analysis was carried out for each valve type.
To assess the economic impact at national level, we used public information from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Valve for Life initiative, which recorded a TAVR penetration rate in Portugal of 54 procedures per million population in 2017.26
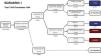
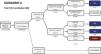
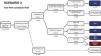
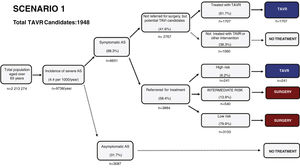
Estimated future demand for transcatheter aortic valve replacement in PortugalTo estimate future demand for TAVR, we collected information on national demographics, disease prevalence and incidence, and data on the natural history of disease. A recent meta-analysis calculated a prevalence of severe AS of 0.8% in individuals aged >65 years, and a yearly incidence of 4.4 (95% confidence interval 3.0-6.1) per 1000 individuals.27 Information on the number of patients referred for treatment was collected from previous epidemiological data27–29 and risk categorization was based on the Society of Thoracic Surgeons dataset.30 Using this information, a clinical decision tree was built to estimate the annual number of TAVR candidates, as previously reported.27
Three different scenarios were constructed to account for the expansion in clinical indications for TAVR from inoperable patients to patients with high,12 intermediate,13,14,31 and low risk.15,16 In scenario 1, we projected that TAVR will be used only according to indications in the current guidelines.11 In scenario 2, we anticipated that TAVR will become the therapy of choice in most patients aged >65 years with intermediate surgical risk.13,14,31 Scenario 3 assumed that, with continuing technological advances and in accordance with the most recent clinical trials, TAVR will also become standard therapy in patients at low risk aged >75 years.15,16,32
ResultsCost of the transcatheter aortic valve replacement procedure in PortugalTable 1 details the costs of the TAVR procedure. The total cost of TAVR with an SEV was 22134.5 euros, while with a BEV it was 23321.5 euros. Most of this figure was due to the cost of the valve itself (74.5% and 81.5% of the total cost, respectively). Although the cost of the BEV is higher (19000 euros vs. 16500 euros), the valve kit includes some additional material which reduces this price difference. Moreover, the risk of complications was slightly lower with the BEV (296.2 euros vs. 337.4 euros), although this was insufficient to reduce the total cost, because the overall risk of complications was low. It is noteworthy that although the procedure is technically demanding, the human resources costs were very low, representing only 1.1% of the total cost of the procedure.
Current economic impact of the procedure at local and national levelIn 2017, Centro Hospitalar de Vila Nova Gaia performed 120 TAVR procedures: 62 with SEV and 58 with BEV. Therefore, TAVR had a local economic impact of 2 724 986 euros in 2017. At a national level, according to the EAPCI Valve for Life Initiative, 550 TAVR procedures were performed in the country in 2017,26 at a total estimated cost of 12500000 euros.
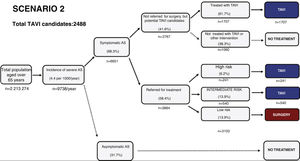
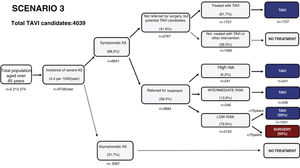
Estimating the future demand for transcatheter aortic valve replacement in PortugalTo estimate the future demand for the technology, three scenarios were constructed. In scenario 1 the estimated number of TAVR candidates was 1948 procedures per year, corresponding to 189 procedures/million population (Figure 2). If TAVR is expanded to patients with intermediate risk (scenario 2, Figure 3) the number of TAVR candidates will increase by 28% to 2488 candidates (241 procedures per million). Finally, if TAVR expands to scenario 3 (Figure 4), the increase will be 107%, corresponding to 4039 TAVR candidates (391 procedures/million).
Estimated number of candidates eligible for transcatheter aortic valve replacement in Portugal according to scenario 3, in which the clinical indication of TAVR is expanded to include low-risk patients at low risk aged over 75 years. AS: aortic stenosis; TAVR: transcatheter aortic valve replacement.
If these three scenarios were applied with current procedure costs, the economic impact would increase to 43770586 euros in scenario 1, 55904116 euros in scenario 2 and 90754310 euros in scenario 3.
It is also important to mention the need to improve the limited installed capacity to respond to scenarios 2 and 3, due not only to the predicted number of facilities needed, but also to the future demand for interventional cardiologists specializing in structural valve disease.
DiscussionIn this study, the real cost of the TAVR procedure in Portugal was calculated for the first time. This cost was slightly higher for BEV than for SEV, even after considering the risk of complications. The current economic impact of TAVR in Portugal is high, with an estimated cost of 12 500 000 euros in 2017. This was however a retrospective study, and the estimated costs refer to the mean expenses per patient, instead of a prospective analysis of the total cost for each patient.
All projections and economic scenarios show that in the near future this economic impact will increase significantly. The current TAVR reimbursement model is inappropriate for the situation, and the creation of an independent line of TAVR reimbursement is therefore justified.
Current costs and economic impact of transcatheter aortic valve replacement in PortugalThe total cost of TAVR hospitalization in Portugal (∼23000 euros) is significantly lower than in the US or even in central European countries.19,20,33,34 In Portugal, most of the cost of the procedure (75-80%) is driven by the cost of the material, a proportion that is higher than other countries, such as in France (65%).35 In absolute terms, the prices of the valves are not very different from the Netherlands19 (17590 euros) or France35 (19500 euros), but are significantly lower than in the US36 (26390 euros). Given the high costs of the procedure and the prospect of increasing numbers of candidates in the near future, it is important to discuss the reimbursement model for public hospitals and to implement strategies to improve it and simultaneously reduce costs, to enable more patients to be treated. It should be borne in mind that the aim of the present study was not to compare data on TAVR with surgical valve replacement or to determine the incremental cost of TAVR to the national healthcare system relative to surgery.
First, considering that the price of the valve itself is the most important driver of the total cost, saving strategies should concentrate on decreasing their price per unit. This can be accomplished by central negotiation of the price to be paid for the valves, by acquiring them at national level, instead of the hospital level.37
Although in our study the price of the SEV was slightly lower, both options should be kept available, so that the main driver of this choice can be the patient's clinical and anatomical characteristics,23 to reduce the risk of complications.
Secondly, TAVR should be performed only in high-volume centers that can further streamline the procedure with dedicated teams. Several studies have shown that this approach can reduce procedure time, decrease costs and reduce complications.38,39
Third, in this study we observed that the use of hospital facilities (especially stay in the cardiac intensive care unit) is the second most important contributor to the overall cost. To lower this cost, it is now possible to design and implement fast-track TAVR protocols in selected patients that allow very short ICU stays (<24hours), decrease total length of hospital stay (<4 days) and decrease costs.40,41 All of these ways to reduce costs should be discussed for application in the Portuguese healthcare system.
Planning the future use of transcatheter aortic valve replacement and its implications for the reorganization of the healthcare systemSeveral studies have analyzed the cost-effectiveness of TAVR, with differing results according to the clinical setting.42 In inoperable patients, almost all analyses show that TAVR has an acceptable cost to society,33 depending on the comorbidities of the treated population.43–45 In high- or intermediate-risk patients, clinical trial results show short-term advantages in quality of life and length of hospital stay, with similar survival rates for TAVR and surgical valve replacement.13,14,19 In the latter setting, cost-effectiveness analyses are less favorable, more variable and strongly influenced by methodology, patient characteristics and the jurisdictions and countries where they were applied.42
As shown in Figure 1, the current TAVR penetration rate in Portugal is below the European average,21 which highlights the considerable inequities in access to this technology.
Additionally, the penetration rate in this country is below that expected according to the current TAVR guidelines11 (53 vs. 189/million), which is valuable information for healthcare resource planning, especially to develop policy on investment in new equipment, to predict future needs in human resources, and to plan budgetary requirements.
This number may increase significantly in the near future if TAVR indications are expanded to patients at intermediate or even low risk, as estimated in scenarios 2 and 3. This change has already occurred in some countries, including Germany and Switzerland.17,18
Nevertheless, at current valve prices, it is highly unlikely that TAVR will be considered a cost-effective treatment in these patients.36 It is expected that with more competition and more patients treated, prices will decrease significantly in the near future, as has been seen with other new cardiovascular technologies.46 However, until rigorous cost-effectiveness analysis is available in these lower-risk patients, it is not recommended that current TAVR indications in Portugal should be expanded.
Health policy recommendations: models of transcatheter aortic valve replacement reimbursement adjusted to the Portuguese healthcare systemIn Portugal there is no specific diagnosis-related group (DRG) for the TAVR procedure, and hospitals are only reimbursed with the DRG associated with traditional surgery (2316.48 euros per patient), which covers only 10.3% of the total cost of TAVR. This means that the remainder of the procedural cost is assumed by the local hospital, using its overall budget or by increasing the hospital's deficit. Some hospitals have accordingly imposed a cap on the number of procedures per year, but this process has been arbitrary and variable, and causes significant regional inequalities in access to the technology.47
Different countries have implemented various models of TAVR reimbursement. For example, in Germany and Switzerland, TAVR is reimbursed using a specific DRG. It is known that hospital financing using DRGs can improve efficiency and transparency and expand hospital activity,48–50 but can also increase total healthcare costs, especially when the DRG funding follows a linear model. In fact, there is a direct association between TAVR penetration rates and reimbursement by DRG.22 However, this system can also have unintended effects. For example, in Germany, the TAVR DRG is 2.3 times higher than the DRG of surgical replacement (∼35000 euros vs. ∼15000 euros),51 which has created an incentive to perform TAVR instead of surgery in lower-risk patients, as recently reported.17,18
Study limitationsIn this study, data was collected retrospectively, in contrast to most economic analyses in TAVR, in which costs are usually calculated based on data collected from randomized clinical trials on TAVR.20,34 However, it is known that economic studies based on well-performed observational studies are equally valuable and can more accurately reflect real costs, adjusted for each country.52
To calculate procedural costs, the authors used data from a single TAVR center. Although we tried to include other centers, hospital administrations did not readily deliver the unit costs needed to perform this study. To obtain a clear picture of health expenditures in Portugal on different medical technologies and procedures, there should be more transparency regarding this type of data.
In Portugal, all centers performing TAVR are tertiary hospitals with similar characteristics,53 even though there may be slight differences in the personnel involved in the procedure, with variable ratios of radiology technicians to nurses. Salary costs are similar and therefore we do not expect there to be major differences between hospitals in the cost of the procedure.
ConclusionsIn Portugal, TAVR is associated with a significant economic impact, which is expected to increase in the near future according to all scenarios. There is an urgent need to implement a new TAVR reimbursement model, which should be able to improve patient access to the technology and to increase efficiency, without compromising the sustainability of the healthcare system.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the TAVR National Registry Investigators.