Clinical trials have shown that functional assessment of coronary stenosis by fractional flow reserve (FFR) improves clinical outcomes. Intravascular ultrasound (IVUS) complements conventional angiography, and is a powerful tool to assess atherosclerotic plaques and to guide percutaneous coronary intervention (PCI). Computational fluid dynamics (CFD) simulation represents a novel method for the functional assessment of coronary flow. A CFD simulation can be calculated from the data normally acquired by IVUS images. A case of coronary heart disease studied with FFR and IVUS, before and after PCI, is presented. A three-dimensional model was constructed based on IVUS images, to which CFD was applied. A discussion of the literature concerning the clinical utility of CFD simulation is provided.
Estudos clínicos demonstraram que a avaliação funcional da doença coronária com fio de pressão e determinação da reserva fracionada de fluxo (fractional flow reserve - FFR) melhora a evolução clínica. O estudo por ultra-som intravascular (IVUS) complementa a angiografia convencional, e é uma ferramenta poderosa para a avaliação das placas ateroscleróticas orientando a intervenção coronária percutânea (ICP). A simulação computorizada da dinâmica de fluidos (CFD), representa um novo método que permite avaliar funcionalmente o fluxo coronário. A CFD pode ser calculada a partir dos dados normalmente obtidos por IVUS. É apresentado um caso de doença coronária estudada com FFR e IVUS, antes e após a ICP. Foi construído um modelo tridimensional com base nas imagens obtidas por IVUS e avaliado com a técnica de CFD. É apresentada uma discussão bibliográfica sobre a utilidade clínica da simulação com CFD.
Randomized studies have shown that functional assessment of coronary stenosis by fractional flow reserve (FFR) improves clinical outcomes and reduces unnecessary revascularizations.1,2 Intravascular ultrasound (IVUS) has been in daily use in catheterization laboratories for several years. IVUS complements conventional angiography, and is the gold standard for assessment of atherosclerotic plaques. It also provides live information on the size of the healthy vessel and lesion characteristics, which increases the accuracy of endoluminal treatment, thereby improving the outcomes of percutaneous coronary intervention (PCI).3 However, the relationship between anatomic data from IVUS and functional assessment of ischemia is the subject of debate.4 Computational fluid dynamics (CFD) simulation is a novel method for the prediction of blood flow, pressure gradients and functional assessment of specific coronary lesions.5 These techniques can be applied to functional assessment of coronary flow from the data normally acquired by IVUS, by three-dimensional (3D) reconstruction of models of their images and application of CFD. A case with coronary artery disease assessed by FFR and IVUS is presented in which 3D reconstruction and CFD were subsequently applied to the IVUS images.
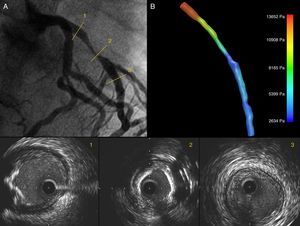
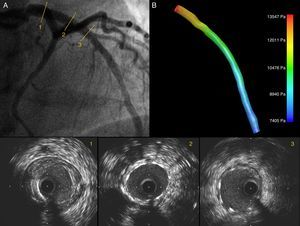
Case reportA 61-year-old male patient with a history of hypertension was admitted in 2011 due to exertional angina for one month. Coronary angiography showed a significant lesion in the proximal left anterior descending artery, an image of myocardial bridging in the mid segment, and a significant lesion in the distal segment. FFR was performed with intravenous administration of adenosine 140 μg/kg/min, yielding a value of 0.40. IVUS detected a calcified lesion in the proximal segment with a minimal luminal area of 2.32 mm2 and a length of 29.76 mm (Figure 1). A 3.5 mm×28 mm drug-eluting stent was implanted directly in the proximal lesion at 16 atm for 5 seconds. Post-PCI IVUS showed correct stent expansion and apposition (Figure 2). FFR was repeated, obtaining a value of 0.80. Medical treatment for the distal lesion was decided. The patient was discharged two days later without complications.
Coronary angiography showing a significant lesion in the proximal left anterior descending (LAD) artery (A). IVUS images show the LAD ostium without significant lumen involvement and an eccentric calcified lesion (1); in the mid segment a concentric lesion with severe calcification is visible (2), and the distal segment has a fibrous, concentric, non-significant plaque (3); (B) 3D IVUS reconstruction of the LAD with pressure gradients obtained through CFD.
Angiography showing a good angiographic result after PCI of the LAD (A); IVUS images show good expansion and correct stent apposition (2, 3). The LAD ostium is preserved; (B) 3D IVUS reconstruction of the LAD with pressure gradients obtained through CFD showing a reduction in pressure drop after PCI.
Three-dimensional intravascular ultrasound reconstruction and computational fluid dynamics implementation
Three-dimensional IVUS reconstruction was performed using the publicly available and validated software IVUSAngio Tool.6 ANSYS release 14.5 software was used for CFD simulation in accordance with the manufacturer's User's Guide.7 A linear model based on stream pressure was used.8 Blood was treated as a homogeneous Newtonian fluid with a dynamic viscosity of 3.5 mPa/s and a density of 1050 kg/m3. Blood flow was assumed to be laminar and incompressible. A constant and specific uniform model for inlet profile was specified. Coronary blood flow was calculated for each model by measuring the angiographic frames required for contrast to pass from the inlet to the outlet of the reconstructed segment, the 3D volume of the lumen and the angiographic frame rate. The arterial wall was considered rigid. Pressure used in the inlet was 13332 Pa, assuming maximal hyperemia with zero resistance to outlet flow.
In the pre-PCI model, a volume of 295.3 mm3 and a flow of 0.55 ml/s with a pressure gradient of 11018 Pa were obtained. In the post-PCI model a volume of 361.5 mm3 with an improved flow of 1.88 ml/s, and a reduction in the pressure gradient of 6142 Pa were obtained. The time for creating the 3D models and implementing the CFD simulation was 185 and 135 minutes respectively.
DiscussionThis study exemplifies the value of CFD applied to 3D IVUS reconstruction, as seen in other studies on the use of CFD in diagnosis.5,9 Of these, the most important was probably DISCOVER-FLOW, in which Koo et al. described the relationship between FFR obtained by 3D reconstruction of coronary computed tomography angiography (CTA) and FFR measured in the catheterization laboratory in patients with stable coronary artery disease and intermediate lesions with greater than 50% stenosis on CTA. The correlation they found between the two methods was 0.72 (p<0.05), improving the diagnostic accuracy of coronary CTA to predict functionally significant lesions of 50–80%.5 Morris et al. recently reported similar findings, using 3D reconstruction models of angiographic images.9 However, although the correlation between the results obtained from CFD and invasive angiography is good, the technique has limitations. Firstly, 3D reconstruction of any exam, whether CT, angiography, or IVUS, does not exactly represent the structure under study, as it is impossible to recreate human anatomy in this way. Moreover, CFD simulation cannot fully predict the characteristics of physiologic processes, which will lead to small variations in the results.10
In conclusion, we believe that the use of 3D IVUS reconstruction associated with CFD can improve the diagnostic accuracy of IVUS for the functional assessment of coronary lesions.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.