Sympathetic renal denervation (RDN) was developed as a treatment for the management of patients with resistant hypertension. This procedure may have a positive impact on hypertension-related target organ damage, particularly renal disease, but the evidence is still limited.
ObjectiveTo assess the impact of RDN on the albumin-to-creatinine ratio (ACR) at 12-month follow-up.
Methods and ResultsFrom a single-center prospective registry including 65 patients with resistant hypertension undergoing renal denervation, 31 patients with complete baseline and 12-month follow-up blood pressure (BP) measurements (both office and 24-h ambulatory blood pressure monitoring [ABPM]) and ACR were included in the present study. Mean age was 65±7 years, 52% were female, most (90%) had been diagnosed with hypertension for more than 10 years, 71% had type 2 diabetes and 33% had vascular disease in at least one territory. Mean estimated glomerular filtration rate was 73.6±25.1 ml/min/1.73 m2 and 15 patients (48%) had an ACR >30 mg/g. After 12 months, 22 patients were considered BP responders (73%). ACR decreased significantly from a median of 25.8 mg/g (interquartile range [IQR] 9.0-574.0 mg/g) to 14.8 mg/g (IQR 4.5-61.0 mg/g, p=0.007). When the results were split according to systolic BP responder status on ABPM, we found a significant reduction in responders (from 25.6 mg/g [IQR 8.7-382.8 mg/g] to 15.9 mg/g [IQR 4.4-55.0 mg/g], p=0.009), and a numerical decrease in the non-responder subgroup (from 165.0 mg/g [IQR 8.8-1423.5 mg/g] to 13.6 mg/dl [IQR 5.7-1417.0 mg/g], p=0.345).
ConclusionsBesides significant reductions in blood pressure (both office and 24-h ABPM), renal denervation was associated with a significant reduction in ACR, a recognized marker of target organ damage.
A desnervação simpática renal (RDN) foi desenvolvida como uma forma de tratamento para os doentes com hipertensão arterial resistente (R-HTN). Este procedimento poderá ter um impacto favorável nas lesões de órgão alvo relacionadas com a hipertensão, nomeadamente a doença renal, no entanto, a evidência disponível ainda é escassa.
ObjetivoAvaliar o impacto da RDN no rácio albumina-creatinina (ACR) aos 12 meses de seguimento após RDN.
Métodos e resultadosRegisto prospetivo de centro único incluindo 65 doentes com R-HTN submetidos a RDN, dos quais 31 doentes com avaliação basal e a um ano completa da pressão arterial (na consulta e na monitorização ambulatória [ABPM]) e da ACR foram incluídos no presente estudo. A idade média foi de 65±7 anos, 52% do sexo feminino. A maioria da população tinha diagnóstico de HTN há >10 anos, 71% tinha diabetes tipo 2 e 33% tinham doença vascular em pelo menos um território. A taxa de filtração glomerular estimada foi de 73,6±25,1 ml/min/1,73 m2 e 48% (15 doentes) tinham uma ACR>30 mg/g. Aos 12 meses de seguimento, 22 doentes foram considerados respondedores na pressão arterial (73%). A ACR teve uma descida significativa de uma mediana de 25,8 mg/g (IQR 9,0-574,0 mg/g) para 14,8 mg/g (IQR 4,5-61,0 mg/g, p=0,007). Quando os resultados foram divididos em subgrupos, de acordo com o estado de respondedor à pressão arterial na ABPM, verificou-se uma redução significativa nos respondedores (de 25,6 mg/g [IQR 8,7-382,8 mg/g] para 15,9 mg/g [IQR 4,4-55,0 mg/g], p=0,009), e uma tendência no subgrupo de não respondedores (de 165,0 mg/g [IQR 8,8-1423,5 mg/g] para 13,6 mg/dl [IQR 5,7-1417,0 mg/g], p=0,345).
ConclusãoPara além da descida significativa da pressão arterial (quer na consulta quer na monitorização ambulatória de 24 h), a desnervação renal associou-se a uma redução significativa da ACR, um reconhecido marcador de lesão de órgão alvo na hipertensão arterial.
Cardiovascular disease is the leading cause of morbidity and mortality in developed countries and hypertension is one of its most important risk factors.1 Some hypertensive patients have drug-resistant hypertension and are at a higher risk of events.2,3 Besides clinical events, assessment of target organ damage can provide earlier insights into the biological impact of hypertension. For several years, albuminuria has been recognized as an indicator of cardiovascular risk, although the pathophysiology behind this association is still not fully understood.4–6
In recent years sympathetic renal denervation (RDN) has been developed as a treatment for the management of patients with resistant hypertension7,8 and it may have a positive impact on hypertension-related target organ damage. An example is recently published reports of reductions in left ventricular hypertrophy after RDN.9–11 The kidney is also an important organ in this context, but evidence on the effect of RDN on proteinuria is still limited and results are conflicting.12,13 The aim of the present study was to assess the impact of RDN on the albumin-to-creatinine ratio (ACR) at 12-month follow-up.
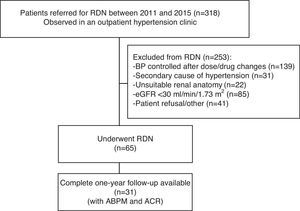
MethodsStudy design and patient populationFrom a single-center prospective registry including 318 patients with resistant hypertension referred for RDN between July 2011 and April 2015, we included for the purpose of the present study 31 patients with complete information on blood pressure (BP) measurements (both office and 24-h ambulatory blood pressure monitoring [ABPM]) at baseline and 12 months, transthoracic echocardiogram and renal function (creatinine clearance and ACR), out of 65 patients who were considered good candidates and underwent RDN (Figure 1).
Flowchart of patient selection. Of the total number of patients observed in an outpatient hypertension clinic (n=318), 253 were excluded for various reasons (see main text) and 65 underwent renal denervation. Of these 65, complete 12-month follow-up with ambulatory blood pressure monitoring and transthoracic echocardiographic data were available in 31 patients. ABPM: 24-h ambulatory blood pressure monitoring; ACR: albumin-to-creatinine ratio; BP: blood pressure; eGFR: estimated glomerular filtration rate; RDN: renal denervation.
The details of this patient population have been previously described.11,14 Briefly, all patients who underwent RDN were aged over 18 years, with persistent office systolic blood pressure (SBP) above 160 mmHg even after optimal antihypertensive therapy (at least three drugs, including a diuretic). Before RDN all patients were studied for secondary causes of hypertension and visited regularly (for at least six weeks) in order to ensure drug regime optimization and full compliance with medical treatment.
Demographic, clinical, anthropometric, laboratory and procedural data were recorded and stored in a dedicated database and written informed consent was obtained from all patients. The study was approved by the ethics committee of Hospital de Santa Cruz and Nova Medical School, Lisbon, Portugal.
Blood pressure measurement and definition of respondersOffice BP readings were measured in a seated position, after a 5 min rest (in accordance with the European guidelines for the management of arterial hypertension), using an Omron HEM-907 semiautomatic oscillometric sphygmomanometer (Omron Healthcare, USA).
At baseline, before RDN, BP measurements were taken in both arms and the arm with the higher BP was selected for all subsequent readings. The mean of three measurements was used for analysis. An ABM monitor (Spacelabs Healthcare, USA) was used for 24-h ABPM assessment.
Patients with a decrease of 10 mmHg or more in office SBP or of 2 mmHg or more in 24-h ABPM SBP at 12-month follow-up were considered to be BP responders to RDN.11,15
Renal function and albuminuria measurementCreatinine clearance was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula. Albuminuria was obtained in spot urine and measured using the ACR, expressed in mg/g, which is equivalent to 24-h albumin excretion in mg/day. ACR values were acquired before RDN (at baseline) and at 12-month follow-up.
Renal denervation procedureRDN procedures were performed using mild anesthesia (propofol and remifentanil) for sedation and pain control. An activated clotting time of 250-300 s was obtained with unfractionated heparin. After femoral artery access, abdominal aortography and selective renal artery angiography were performed to confirm anatomic eligibility. In all cases, the femoral access site was closed using a sealing device (Angio-Seal®, St. Jude Medical, USA).
Denervation was performed using the following radiofrequency systems: Symplicity® (n=25), EnligHTN® (n=4) and OneShot® (n=2), in accordance with standard techniques.
Statistical analysisContinuous variables are reported as mean ± standard deviation. Normality was tested with the Kolmogorov-Smirnov test and/or visual assessment of a Q-Q plot. Normally distributed variables were compared between baseline and 12-month follow-up using a paired Student's t test, or a Wilcoxon matched-pairs test if not normally distributed. Discrete variables are expressed as frequencies and percentages. Statistical comparisons of characteristics at baseline and at follow-up were performed using the chi-square test or Fisher's exact test, as appropriate, for categorical variables and the paired Student's t test for continuous variables. A two-tailed p value <0.05 was considered as statistically significant.
SPSS® version 21.0 (IBM SPSS Inc, Chicago, IL) was used for data processing and statistical analysis.
ResultsPatient characteristicsA total of 318 patients were observed in an outpatient hypertension clinic between 2011 and 2015. Of these, 253 were excluded due to: (a) BP control being achieved after dose and/or drug changes (n=139); (b) a secondary cause of hypertension (n=31); (c) renal anatomy considered unsuitable for RDN on computed tomography angiography; (d) estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2; (e) patient refusal after discussion of the expected benefits and risks. A total of 65 patients were considered good candidates and underwent RDN. Of these, the first 31 with complete data on blood and urine samples, office BP and 24-h ABPM, and transthoracic echocardiogram at both baseline and 12-month follow-up were included in the present analysis (Figure 1). Data on left ventricular mass and function have been reported elsewhere.11
Mean age was 65±7 years, all patients were Caucasian and 48% (n=14) were male. Regarding cardiovascular risk factors, 71% had type 2 diabetes, 68% were obese (mean body mass index 32±6 kg/m2), 68% had dyslipidemia and only one patient was an active smoker. Ten patients (33%) had manifestations of vascular disease (mainly coronary artery disease). Mean eGFR was 76.4±24.7 ml/min/1.73 m2 and five patients had chronic kidney disease (eGFR <60 ml/min/1.73 m2) (Table 1). At baseline, median ACR was 25.8 (interquartile range [IQR] 9.0-574.0) and 15 (48%) patients had ACR >30 mg/g.
Baseline characteristics and renal denervation procedures.
| Demographic and clinical variables | |
| Age (years) | 65±7 |
| Male (%) | 15 (48.4) |
| Caucasian (%) | 31 (100) |
| Weight (kg) | 86±16 |
| Height (m) | 1.65±0.1 |
| BMI (kg/m2) | 31.8±5.5 |
| Obesity (%) | 21 (67.7) |
| Atrial fibrillation (%) | 1 (3.2) |
| Previous stroke (%) | 2 (6.5) |
| Type 2 diabetes (%) | 22 (71) |
| Dyslipidemia (%) | 21 (67.7) |
| Smoking (%) | 1 (3.2) |
| Sleep apnea (%) | 5 (19.1) |
| eGFR (ml/min/1.73 m2) | 76.4±24.7 |
| CKD (%) | 5 (16.1) |
| Hypertension >10 years (%) | 28 (90.3) |
| Coronary artery disease (%) | 10 (32.3) |
| Any vascular disease (%) | 11 (35.5) |
| RDN procedure | |
| Mean no. of RF applications, right renal artery | 5.1±1.3 |
| Mean no. of RF applications, left renal artery | 5.7±1.1 |
| Mean no. of RF applications per patient | 10.8±2.3 |
BMI: body mass index; CKD: chronic kidney disease (eGFR <60 ml/min/1.73 m2); eGFR: estimated glomerular filtration rate; RDN: renal denervation; RF: radiofrequency.
Most patients (90%) had known hypertension for at least 10 years and were treated at baseline with a mean of 5.8 anti-hypertensive drugs, corresponding to a mean of 5.5 different drug classes. Of note, 74% were treated with spironolactone. Details on antihypertensive medication at baseline and follow-up are presented in Table 2.
Antihypertensive medication.
| Baseline | 12 months | p | |
|---|---|---|---|
| Mean no. of antihypertensive drugs | 5.8±1.1 | 5.0±1.2 | 0.002 |
| Mean no. of drug classes | 5.5±0.9 | 4.9±1.1 | 0.015 |
| ACE inhibitors | 19 (61.3) | 17 (54.8) | 0.688 |
| ARBs (%) | 19 (61.3) | 18 (58.1) | 1.0 |
| Beta-blockers (%) | 26 (83.9) | 27 (87.1) | 1.0 |
| Calcium channel blockers (%) | 30 (96.8) | 21 (67.7) | 0.012 |
| Diuretics (%) | 27 (87.1) | 24 (77.4) | 0.727 |
| Spironolactone (%) | 23 (74.2) | 26 (83.9) | 0.453 |
| Sympatholytics (%) | 22 (71) | 19 (61.3) | 0.508 |
| Aliskiren | 4 (12.9) | 0 | 0.046 |
ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blockers.
Mean office SBP and diastolic BP (DBP) at baseline were 176±24 mmHg and 90±14 mmHg, respectively, and mean heart rate was 73±11 bpm. On 24-h ABPM, mean SBP and DBP were 150±20 mmHg and 83±10 mmHg, respectively (Table 3).
Blood pressure, heart rate and albumin-to-creatinine ratio before and 12 months after renal denervation.
| Baseline | 12 months | p | |
|---|---|---|---|
| Office SBP (mmHg) | 176±24 | 149±13 | <0.001 |
| Office DBP (mmHg) | 90±14 | 79±11 | <0.001 |
| Heart rate (bpm) | 73±11 | 70±11 | 0.261 |
| ABPM SBP (mmHg) | 150±20 | 132±14 | <0.001 |
| ABPM DBP (mmHg) | 83±10 | 74±9 | <0.001 |
| ABPM pulse pressure (mmHg) | 67±18 | 58±13 | 0.001 |
| ABPM mean BP (mmHg) | 105±9 | 95.3±8.4 | <0.001 |
| ABPM heart rate (bpm) | 67.6±9.1 | 65.5±9.5 | 0.090 |
| ABPM SBP respondersa (%) | - | 26 (83.9) | - |
| Office SBP respondersb (%) | - | 22 (71) | - |
| eGFR (ml/min/1.73 m2) | 73.6±25.1 | 72.5±25.1 | 0.711 |
| ACR (mg/g) | 25.8 (9.0-574.0) | 14.8 (4.5-61.0) | 0.007 |
| ACR in ABPM BP responders (mg/g) | 25.6 (8.7-382.8) | 15.9 (4.4-55.0) | 0.009 |
| ACR in ABPM BP non-responders (mg/g) | 165.0 (8.8-1423.5) | 13.6 (5.7-1417.0) | 0.345 |
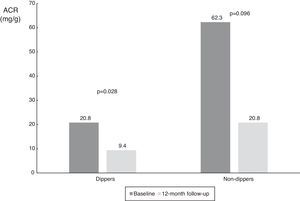
| ACR in ABPM dippers (mg/g) | 20.8 (6.8-290.0) | 9.4 (3.7-41.1) | 0.028 |
| ACR in ABPM non-dippers (mg/g) | 62.3 (9.1-852.3) | 20.8 (9.3-197.1) | 0.096 |
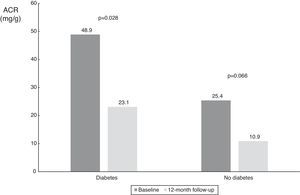
| ACR in diabetic patients (mg/g) | 48.9 (9.1-1116.3) | 23.1 (4.3-123.8) | 0.028 |
| ACR in non-diabetic patients (mg/g) | 25.4 (5.2-68.6) | 10.9 (3.4-20.8) | 0.066 |
ABPM: 24-h ambulatory blood pressure; ACR: albumin to creatinine ratio; BP: blood pressure; bpm: beats per minute; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; SBP: systolic blood pressure.
At 12-month follow-up mean SBP had decreased from 176±24 to 149±13 mmHg (p<0.001) and DBP from 90±14 to 79±11 mmHg (p<0.001). These changes were consistent with the results of 24-h ABPM, in which mean SBP decreased from 150±20 to 132±14 mmHg (p<0.001) and mean DBP from 83±10 to 74±9 mmHg (p=0.001). At 12-month follow-up, 71% of patients were considered office SBP responders and 84% were considered ABPM SBP responders (Table 3). During this period there was also a reduction in the number of antihypertensive drugs and classes taken; the number of drugs decreased from 5.8±1.1 to 5.0±1.2 (p=0.002) and drug classes from 5.5±0.9 to 4.9±1.1 (p=0.02) (Table 2).
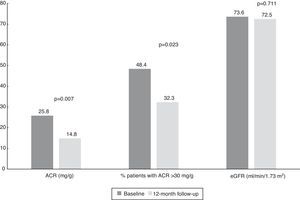
Changes in albumin-to-creatinine ratio after renal denervation and relation with blood pressure controlAt baseline, median ACR was 25.8 mg/g (IQR 9.0-574.0 mg/g) and 48.4% of patients (n=15) had an ACR >30 mg/g. We found a significant reduction at 12-month follow-up to a median of 14.8 mg/g (IQR 4.5-61.0 mg/g, p=0.007) (Table 3).
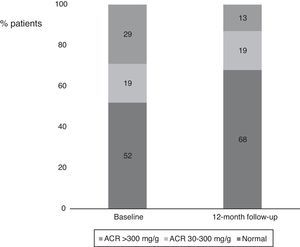
Interestingly, we also found a significant reduction in the percentage of patients with ACR >30 mg/g between baseline and 12-month follow-up (Figure 2). Considering patients with any ACR reduction as ACR responders, 77.4% (n=24) of patients were ACR responders. The distribution of patients across the different classes of urinary albumin excretion also demonstrated a favorable effect (Figure 3).
Values of albumin-to-creatinine ratio and estimated glomerular filtration rate at 12 months after renal denervation. There was a significant reduction in the median values of ACR and the percentage of patients with ACR >30 mg/g, without significant changes in eGFR. ACR: albumin-to-creatinine ratio; eGFR: estimated glomerular filtration rate.
Percentages of patients in the different albumin-to-creatinine ratio subgroups. There was a numerical decrease in the percentage of patients with an ACR >300 mg/g and an increase in patients with normal urinary albumin excretion between baseline and 12-month follow-up. ACR: albumin-to-creatinine ratio.
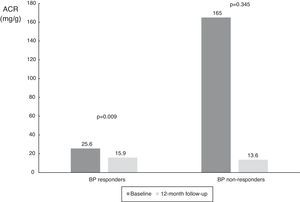
When the results were split according to ABPM SBP responder status, we found a significant reduction in responders (from 25.6 mg/g [IQR 8.7-382.8 mg/g] to 15.9 mg/g [IQR 4.4-55.0 mg/g], p=0.009), and a numerical decrease in non-responders (from 165.0 mg/g [IQR 8.8-1423.5 mg/g] to 13.6 mg/dl [IQR 5.7-1417.0 mg/g], p=0.345), probably due to the small number of patients in this subgroup (Table 3 and Figure 4). The same analysis was performed according to dipper status and the results were similar, with a significant reduction in patients with dipper status on baseline ABPM (Table 3 and Figure 5).
Values of albumin-to-creatinine ratio at 12 months after renal denervation in 24-h ambulatory blood pressure monitoring responder subgroups. There was a significant reduction in median ACR in the BP responder subgroup, and a numerical decrease in non-responders. ACR: albumin-to-creatinine ratio; BP: blood pressure.
Values of albumin-to-creatinine ratio at 12 months after renal denervation according to dipper status on 24-h ambulatory blood pressure monitoring. There was a significant reduction in median ACR in the dipper subgroup, and a numerical decrease in non-dippers. ACR: albumin-to-creatinine ratio.
With regard to diabetic status, patients with diabetes had a higher median baseline ACR and showed a statistically significant decrease (from 48.9 mg/g [IQR 9.1-1116.3 mg/g] to 23.1 mg/g [IQR 4.3-123.8 mg/g], p=0.028); there was also a numerical decrease in ACR in non-diabetic patients (from 25.4 mg/g (IQR 5.3-68.6 mg/g) to 10.9 mg/dl (IQR 3.4-20.8 mg/g), p=0.066), probably also due to the small patient sample in this subgroup (n=9) (Table 3 and Figure 6).
Values of albumin-to-creatinine ratio at 12 months after renal denervation according to diabetic status. There was a significant reduction in median ACR in patients with diabetes, and a numerical decrease in the smaller subgroup of patients without diabetes. ACR: albumin-to-creatinine ratio.
There were four vascular access complications: three hematomas and one femoral pseudoaneurysm. No significant changes in eGFR were seen (Table 3 and Figure 2).
DiscussionThe main findings of our study are: (1) RDN was associated with a significant BP reduction at 12-month follow-up; (2) there was a significant decrease in median ACR, without significant changes in eGFR, and a significant reduction in the percentage of patients with ACR >30 mg/g between baseline and 12-month follow-up; (3) the reduction in ACR was observed in both BP responders and non-responders.
Although the initial results with catheter-based RDN were very promising,7,8,16 the most recent and largest randomized trial, SYMPLICITY HTN-3,15 failed to meet its primary efficacy endpoint, raising doubts about the real biological effect of this treatment. The unexpected negative results of HTN-3 have been extensively discussed and many possible reasons have been put forward, both clinical (mainly related to patient selection) and technical (procedure-related, particularly the number and pattern of radiofrequency applications).17 Another possible factor is how the efficacy of RDN is currently measured, and examining target organ damage may provide a better assessment than BP values. In line with this are the recent results in LV mass and function after RDN, for which several groups have published positive results at six-month follow-up based on both transthoracic echocardiography11,18 and cardiac magnetic resonance imaging (MRI).10 Our group recently reported a significant reduction in left ventricular mass at 12-month follow-up, without correlation with changes in systolic ABPM.11
Another approach to monitoring hypertension-related target organ damage is to calculate ACR, a recognized marker which has been linked to cardiovascular outcomes in several studies on hypertension.4–6 Ott et al.19 found a significant reduction in ACR at six-month follow-up in 59 patients with resistant hypertension (mean 24-h ABPM SBP 156 mmHg, treated with a mean of 5.5 antihypertensive drugs). In contrast with the latter study, we also included patients with normal (<30 mg/g) baseline ACR and therefore our median values are lower that those reported by Ott et al. Of note, even among this mixed population of different ACR baseline profiles, half of whom had normal urinary albumin excretion (51.6% with ACR <30 mg/dl), the mean age (65 years), baseline ABPM SBP (150 mmHg) and mean number of drugs (5.8) were very similar to the study by Ott et al. Our study also included a higher percentage of patients with type 2 diabetes (71%, compared to 51% in Ott et al.’s study). In another recently published single-center study, Verloop et al.13 failed to demonstrate any significant decrease in either LV mass (by cardiac MRI) or urinary albumin excretion, and found only a modest impact on blood pressure. These results are in contrast with previous studies and our results, and may have been due to differences in patient populations. In the study by Verloop et al.,13 the mean age was lower (58 years) and so was the mean number of drugs (4, as opposed to 5.5 in Ott et al. and 5.8 in our study). Other important differences are the much lower prevalence of diabetes (only 15%) and the fact that the authors did not exclude patients with eGFR <45 ml/min/1.73 m2.
One interesting observation in our study is the fact that the reduction in ACR was also found in BP non-responders, although this did not reach statistical significance, probably due to the small size of this patient subgroup. This raises the question of whether RDN, by reducing sympathetic hyperactivity, might have a positive direct effect on glomerular endothelial function independent of the hemodynamic effect derived from blood pressure reduction, since there is a close association between urinary albumin excretion and glomerular endothelium dysfunction and glycocalyx loss.5,20 These two factors may be modulated by autonomic nervous system tone and, in theory, this positive impact on endothelial physiology could be linked to the expected decrease in overall cardiovascular risk that is the ultimate goal of RDN. On the other hand, endothelial dysfunction is only one of several effects of increased sympathetic activity, a common denominator in cardiovascular and renal pathophysiology.21 Finally, the ACR reduction seen in our study should be interpreted in the context of the high cardiovascular risk of patients with resistant hypertension,2,3 and this reduction is expected to help to lower this risk, although no prospective studies have been published on the prognostic impact of RDN on clinical outcomes.
LimitationsThe present study has some limitations that should be mentioned: (1) it is a single-center prospective registry with a small sample size; (2) the physicians following patients after RDN were not blinded, although the most important outcome measurements (24-h ABPM and ACR) were performed by cardiac and laboratory technicians unaware of treatment status; (3) there was no control group or sham procedure; (4) changes were made in antihypertensive therapy during follow-up, which could have influenced reductions in blood pressure and ACR (but the mean number of drugs actually decreased during follow-up, which could lead to underestimation of the benefit of RDN in this daily practice setting).
ConclusionsIn this single-center unblinded study of patients with resistant hypertension undergoing RDN, we found a significant reduction in both office BP and 24-h ABPM which was associated with a significant decrease in median ACR, without significant changes in eGFR. At 12-month follow-up, there was a reduction in the percentage of patients with pathological urinary albumin excretion, and this reduction was independent of BP responder status.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.