Fingolimod, a structural analogue of sphingosine, is the first oral treatment available for multiple sclerosis. The presence of sphingosine-1-phosphate receptors in the sinus and atrioventricular nodes, myocardial cells, endothelial cells and arterial smooth muscle cells is responsible for fingolimod's cardiovascular effects. We provide a comprehensive review of the mechanisms of these effects and characterize their clinical relevance.
O fingolimod, um análogo estrutural da esfingosina, é o primeiro tratamento oral disponível para a esclerose múltipla. A presença de recetores esfingosina-1-fosfato no nódulo sinusal e auriculoventricular, miócitos cardíacos, células endoteliais e células do músculo liso arterial possibilita a ocorrência de efeitos cardiovasculares com o fingolimod. Neste artigo, faz-se uma revisão dos mecanismos dos efeitos cardiovasculares do fingolimod e a caracterização da sua relevância clínica.
atrioventricular block
beats per minute
central nervous system
disease-modifying drug
electrocardiogram
European Medicines Agency
nitric oxide synthase
Evaluate Patient OutComes Study
G protein-coupled inwardly-rectifying potassium channel
heart rate
interferon beta-1a
multiple sclerosis
nitric oxide
sphingosine-1-phosphate
sphingosine-1-phosphate receptor type 1
The treatment of multiple sclerosis (MS) has seen remarkable progress in the last two decades, with increasingly effective disease control, particularly of its relapsing-remitting forms. Several disease-modifying drugs (DMDs) have been approved and some new molecules are reaching the market, expanding the range of available therapeutic options. Treatment selection is therefore becoming more tailored to the patient's clinical profile and necessarily more complex, requiring a thorough knowledge of the relevant pharmacology on the part of the MS-treating physician.
Fingolimod, a structural analogue of sphingosine, is the first oral DMD approved for MS treatment. Its mode of action is innovative. Its active metabolite, formed by in vivo phosphorylation, modulates sphingosine-1-phosphate (S1P) receptors and induces their downregulation on the surface of lymphocytes. This leads to sequestration of primarily naive and memory T lymphocytes within lymph nodes, potentially reducing trafficking of these pathogenic cells into the central nervous system (CNS).1,2 Moreover, as it is highly lipophilic, fingolimod readily crosses the blood-brain barrier and penetrates the CNS,3 and there is increasing evidence of a direct effect on S1P receptors in oligodendrocytes, astrocytes, and neurons.1,4
In the European Union, fingolimod is approved for use as a single-agent DMD in selected patients with highly active relapsing-remitting MS, defined by the European Medicines Agency (EMA) as a second-line therapy after the failure of interferon beta-1a (IFN beta-1a), or as a first-line therapy for patients with rapidly evolving severe disease.5
Fingolimod's efficacy has been established in three phase III clinical trials,6–8 showing not only superior efficacy in reducing relapse rates and improved MRI measures of disease activity compared to placebo and intramuscular IFN beta-1a, but also reduced risk of disability progression compared to placebo. Regarding its safety profile, fingolimod was generally safe and well tolerated, with most adverse events being of mild to moderate severity, including bradycardia and atrioventricular block (AVB), infections, increased liver enzyme levels, hypertension and macular edema.
Nevertheless, in clinical settings, concerns remain about its cardiovascular effects, particularly after the first dose. Further clarification of fingolimod's effects on the cardiovascular system and its underlying mechanisms will enable practicing clinicians to initiate fingolimod in appropriately selected and screened MS patients, maximizing its effectiveness and safety.
Cardiovascular effects of fingolimod: mechanismsS1P is a bioactive lysophospholipid that mediates several physiological functions.4,9 Although the main source of S1P is the erythrocyte, other sources include activated platelets, mast cells, endothelial cells, fibroblasts and the central nervous system.10–13 S1P regulates various cellular responses such as proliferation, differentiation, survival, cytoskeletal reorganization, formation of cytoplasmic prolongations, chemoattraction and motility, intercellular adhesion and formation of cell junctions. It is thus involved in numerous physiological processes, including immunity, vascular and pulmonary smooth muscle tone, endothelial barrier function, and cardiovascular and nervous system function and morphogenesis. Extracellular S1P exerts its effects by binding to five receptors (S1PR1–5) belonging to the family of G protein-coupled receptors.1,14,15
Fingolimod, a structural analogue of sphingosine, is a prodrug that is phosphorylated by platelet sphingosine kinase type 2, in a reaction that does not require platelet stimulation.16–18 The active drug, fingolimod-phosphate, is an agonist of S1PR1, S1PR4 and S1PR5, and at concentrations ten times higher, it is also an agonist of S1PR3.19,20 Binding of fingolimod-phosphate to the receptor has an initial agonist effect, but prolonged receptor internalization and subsequent proteasomal degradation results in a functional antagonism effect. Therefore, in the long term, the drug is an inhibitor of receptor function.21–23
The presence of S1PR1 and S1PR3 in the heart and blood vessels is responsible for the cardiovascular effects of fingolimod.24,25 Specifically, S1PR1 is predominant in the sinus node, atrioventricular node, myocardial cells and endothelial cells, while S1PR2 is predominant in arterial smooth muscle cells, in which S1PR1 and S1PR3 are present in similar concentrations.26–29
In the heart, activation of S1PR1 causes dissociation of the G protein, thus activating acetylcholinergic G protein-coupled inwardly-rectifying potassium channels (GIRKs).30 Activation of these channels leads to potassium efflux, which hyperpolarizes the cell membrane, hindering its depolarization and decreasing automation and excitability.31 In brief, bradycardia occurs by a mechanism similar to the binding of acetylcholine to the muscarinic receptor and to the mechanism of atrioventricular conduction delay. Internalization of S1PR1 is responsible for the transient nature of fingolimod-induced negative chronotropic and dromotropic effects. Both atropine (a muscarinic receptor antagonist) and isoprenaline (a beta-1 and -2 adrenergic receptor agonist) reverse these side effects of fingolimod.32,33
In endothelial cells, activation of S1PR1 induces phosphorylation of protein kinase B and activates nitric oxide synthase (eNOS), both leading to increased nitric oxide (NO) production.34–36 NO relaxes smooth muscle cells, causing vasodilation. Activation of S1PR1 in arterial smooth muscle cells releases calcium from intracellular stores, increasing its intracellular concentration, and leading to myocyte contraction and vasoconstriction. Thus, blood pressure does not vary significantly in the acute phase following fingolimod administration.
Internalization, with consequent functional antagonism, of S1PR1 favors the binding of S1P to S1PR2 and S1PR3. In arterial smooth muscle cells, activation of S1PR2 and S1PR3 opens calcium channels and increases intracellular calcium concentrations, causing contraction. These effects are responsible for the small increase in blood pressure – 2 mm Hg systolic, 1 mm Hg diastolic – observed at two months of treatment with fingolimod (0.5 mg daily), reaching its peak at six months, and remaining stable thereafter.6,7 The clinical significance of this long-term effect on blood pressure is not established.
Other effects of S1PR1 activation in endothelial cells are reorganization of the cytoskeleton and stabilization of cell geometry and intercellular junctions.37 These strengthen the endothelial barrier, reducing its permeability. Again, this effect is transient due to receptor internalization. The opposite effect, increased permeability of the endothelial barrier, follows since activation of S1PR2 and S1PR3 disrupts cell junctions.
In brief, fingolimod may cause transient bradycardia, and in the medium to long term, a small rise in blood pressure. These effects are due to the functional antagonism of S1PR1 within the cardiovascular system. Fingolimod does not change the duration of the QRS complex nor does it prolong the QT interval.38 Fingolimod also has no significant effects on platelet count or function.39
Experimental studies show that fingolimod can stimulate arteriogenesis and prevent reperfusion injury, suggesting future potential therapeutic applications in the cardiovascular field.40–42
Cardiovascular effects of fingolimod: clinical relevanceThe cardiovascular effects of fingolimod have been evaluated in four phase III clinical trials: TRANSFORMS, FREEDOMS, FREEDOMS II, and FIRST.6,7,43–45 The first three aimed at assessing the efficacy of fingolimod compared with placebo or IFN beta-1a. These studies excluded patients with a baseline heart rate (HR) below 55 beats per minute (bpm), or with history of significant heart disease, including second- or third-degree AVB, sinus node disease, ischemic heart disease, heart failure or heart rhythm disorders treated with class III antiarrhythmics (amiodarone or sotalol). The FIRST study was designed to evaluate the cardiovascular safety of fingolimod at a daily dose of 0.5 mg, and enrolled individuals more similar to real-world patients, such as those with diabetes, asthma and cardiac risk factors, including those undergoing beta-blocker therapy and patients with baseline HR between 45 and 54 bpm, history of Mobitz type I AVB, symptomatic recurrent bradycardia or positive tilt test.
The FREEDOMS, TRANSFORMS, and FREEDOMS II trials assessed the vital signs HR and blood pressure before the first fingolimod dose and every hour thereafter up to six hours. Two electrocardiograms (ECGs) were recorded, one at baseline and one six hours after the first dose. In the FIRST trial, only a subgroup of patients underwent clinical monitoring during the first six hours after the first fingolimod dose.
Effects on heart rateA transient and dose-dependent HR reduction can be observed after the first dose of fingolimod, both in patients with MS and in apparently healthy individuals. However, this effect decreases with continued treatment, returning to baseline HR after approximately one month (Figure 1).
Effects of fingolimod on heart rate: pooled analysis of the FREEDOMS, FREEDOMS II and TRANSFORMS studies. Results are expressed as mean ± standard deviation. The inserted table shows the minimum heart rate (HR) recorded in the first six hours after the first dose of fingolimod. F: fingolimod; IFN: interferon; IFN beta-1a: interferon beta-1a. Adapted from DiMarco et al.46
Pooled analysis of the FREEDOMS, FREEDOMS II, and TRANSFORMS trials showed that the mean maximum HR reduction was 8 bpm for a daily dose of 0.5 mg. HR nadir was typically reached at 4–5 (always before 12) hours after the first dose.46 This HR reduction was generally benign (Table 1): on one hand, HR was maintained above 54 bpm in 80% of patients and never dropped below 40 bpm; on the other hand, bradycardia was symptomatic – reported symptoms were generally mild and consisted of dizziness, fatigue, dyspnea or chest pain – in only 0.6% of patients who received the first dose of fingolimod 0.5 mg. Finally, in the majority of cases, bradycardia resolved spontaneously, without the need for intervention or treatment discontinuation.
Characteristics of bradycardia and atrioventricular conduction disturbances induced by the first dose of fingolimod: pooled analysis of the FREEDOMS and TRANSFORMS trials.
| Bradycardia (%) | Fingolimod 0.5 mg/day (n=1212) | Fingolimod 1.25 mg/daya (n=1219) | Placebo (n=773) | IFN beta-1a (n=431) |
|---|---|---|---|---|
| Bradycardia symptoms | ||||
| All | 0.6 | 2.1 | 0.1 | 0.0 |
| Mild | 0.4 | 1.2 | 0.1 | 0.0 |
| Moderate | 0.2 | 0.7 | 0.0 | 0.0 |
| Severe | 0.0 | 0.2 | 0.0 | 0.0 |
| Treated bradycardia | 2.9 | 5.2 | 0.0 | 0.0 |
| Drug discontinued permanently | 0.1 | 0.4 | 0.3 | 0.0 |
| Atrioventricular conduction disturbancesb(%) | ||||
| First-degree AVB | 4.7 | 9.7 | 1.7 | 2.9 |
| Mobitz type I AVB | 0.2 | 1.0 | 0.0 | 0.0 |
| Second-degree AVB, 2:1 AVB | 0.0 | 0.2 | 0.0 | 0.0 |
| Mobitz type II AVB or third-degree AVB | 0.0 | 0.0 | 0.0 | 0.0 |
AVB: atrioventricular block; IFN beta-1a: interferon beta-1a.
In the FIRST trial, 295 (12.2%) of the 2415 patients treated with fingolimod showed cardiac risk factors and 1219 (50.5%) underwent clinical monitoring for at least six hours after the first dose. In this subgroup of monitored patients, HR nadir occurred at 4–5 hours after the first dose. The mean maximum HR reduction was 7.4 bpm in the 948 patients without cardiac risk factors and 6.5 bpm in the 271 patients with cardiac risk factors. Mean HR before treatment initiation was lower in patients with cardiac risk factors (69 bpm vs. 74 bpm in patients without cardiac risk factors). Among monitored patients, 78 were undergoing treatment with a beta-blocker or calcium channel blocker. The mean maximum HR reduction was 7.3 bpm in these patients and 7.2 bpm in the other 1141 patients. Mean HR before treatment initiation was lower in medicated patients compared to non-medicated patients (69 bpm vs. 73 bpm, respectively). Table 2 shows the incidence of bradycardia in monitored patients.
Incidence of bradycardia and atrioventricular conduction disturbances induced by the first dose of fingolimod in the FIRST trial.
| Bradycardia in the initial six hours after first dose of fingolimoda | |||
|---|---|---|---|
| Patients (n, %) | All (n=1219) | With cardiac risk factors (n=271) | Taking BBs or CCBs (n=78) |
| HR <45 bpm | 16 (1.3) | 12 (4.4) | 2 (2.5) |
| HR ≤40 bpm | 3 (0.2) | 2 (0.7) | 0 (0.0) |
| HR <30 bpm | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Monitoring extended after 6 hours | 40 (3.3) | 15 (5.5) | 3 (3.8) |
| Discharged after hour 7 | 31 (2.5) | 12 (4.4) | 3 (3.8) |
| Holter findings 6 hours before and 6 hours after first dose of fingolimod | |||
|---|---|---|---|
| Patients (n, %) | No cardiac risk factors (n=2120) | With cardiac risk factors (n=295) | Taking BBs or CCBs (n=120) |
| 6 hours before | |||
| Mobitz I | 2 (0.1) | 11 (3.7) | 0 (0.0) |
| 2:1 AVB | 0 (0.0) | 2 (0.7) | 0 (0.0) |
| 6 hours after | |||
| Mobitz I | 18 (0.8) | 13 (4.4) | 0 (0.0) |
| 2:1 AVB | 6 (0.3) | 5 (1.7) | 0 (0.0) |
| Events before and after first dose | 6 (0.3) | 5 (1.7) | 0 (0.0) |
| De novo events only after first dose | 24 (1.1) | 12 (4.0) | 0 (0.0) |
BBs: beta-blockers; CCBs: calcium channel blockers; HR: heart rate.
Criteria for extension of monitoring (any of the following): (1) HR at 6 h ≤80% of baseline HR; (2) HR at 6 h is the nadir; (3) symptomatic bradycardia.
In brief, HR reduction is similar in individuals with or without cardiac risk factors, whether or not treated with bradycardic drugs. Symptoms associated with fingolimod-induced HR reduction are rare, transient, and usually without clinical consequences. The Evaluate Patient OutComes (EPOC) study, a recent multicenter phase IV clinical trial, confirmed these findings.47
Effects on atrioventricular conductionFingolimod may induce clinically benign disturbances in atrioventricular conduction in a small number of patients (Table 1). The PR interval increased on average 4.5 ms for the daily dose of 0.5 mg fingolimod, and 11.3 ms for the 1.25 mg daily dose. First-degree AVB was the most frequent alteration in atrioventricular conduction, showing dose-dependency, but second-degree AVB was rare. The pooled analysis of the FREEDOMS, FREEDOMS II, and TRANSFORMS studies showed that these disturbances in atrioventricular conduction were typically transient and asymptomatic, disappearing in the first 24 hours of treatment.
In the FIRST trial, all patients underwent Holter recording 24 hours before and six hours after treatment initiation. Table 2 shows the incidence of atrioventricular conduction disturbances six hours before and six hours after the first dose of 0.5 mg fingolimod. Overall, the incidence of Mobitz type I AVB and 2:1 AVB after the first dose was 1.3% and 0.5%, respectively. These alterations were more frequent in individuals with cardiac risk factors, but were all asymptomatic and transient, resolving spontaneously, without the need for pharmacological intervention. There was no record of Mobitz type II AVB or complete AVB.
In the FREEDOMS II trial, 1057 patients underwent Holter recording for 24 hours after the first dose of fingolimod.46 Analysis of this population shows that changes in atrioventricular conduction, when present, are more common in the first six hours after the first dose of fingolimod (Table 3).
First atrioventricular conduction abnormality within 24 hours of the first dose of fingolimod: analysis of the FREEDOMS II Holter subgroup.
| Anomaly (%) | Fingolimod 0.5 mg/day (n=351) | Fingolimod 1.25 mg/daya (n=360) | Placebo (n=346) |
|---|---|---|---|
| Any AVB degree ≥2 | |||
| 0–6 hours | 3.1 | 5.0 | 0.0 |
| >6–12 hours | 0.3 | 1.4 | 0.0 |
| >12 hours | 0.6 | 0.3 | 2.0 |
| Second-degree AVB, Mobitz I | |||
| 0–6 hours | 2.6 | 5.0 | 0.0 |
| >6–12 hours | 0.6 | 1.4 | 0.0 |
| >12 hours | 0.6 | 0.3 | 2.0 |
| Second-degree AVB, 2:1 AVB | |||
| 0–6 hours | 1.4 | 2.5 | 0.0 |
| >6–12 hours | 0.0 | 0.8 | 0.0 |
| >12 hours | 0.6 | 0.0 | 0.0 |
AVB: atrioventricular block.
In brief, fingolimod-induced delay in atrioventricular conduction is uncommon and, when present, transient, early and usually without clinical consequences.
Long-term effectsOf the 1272 patients enrolled in the FREEDOMS trial, 920 were included in the extension phase (after 24 months), and 773 completed the study. In this phase, which lasted another two years, patients treated with fingolimod continued therapy and patients previously on placebo were given one of two doses of fingolimod. The incidence of adverse cardiovascular events remained at the level observed in the first two years of the study, suggesting that long-term treatment has no impact on these events. A transient reduction in HR was observed at the beginning of the extension phase, but only in patients who switched from placebo to fingolimod.48 The cardiovascular safety of fingolimod was confirmed in the extension of the TRANSFORMS trial (up to 4.5 years).49 The effects of fingolimod on HR and atrioventricular conduction may recur when treatment is restarted more than two weeks after discontinuation.
Cardiovascular effects of fingolimod: recommendations for safe useThe EMA recommends a six-hour observation period after the first dose of fingolimod in all patients, for assessment of signs and symptoms of bradycardia.5 HR and blood pressure should be recorded prior to the first dose and every hour during the observation period; an electrocardiogram should be performed before the first dose and six hours after. Continuous real-time electrocardiographic monitoring is recommended during these six hours of observation.
The observation period should be extended for at least another two hours if HR at six hours is the nadir value. In these cases, observation should continue until HR rises. The conditions that warrant extending the observation period until at least the next day (until their resolution) are: (1) HR <45 bpm at six hours; (2) corrected QT interval ≥500 ms at six hours; (3) new-onset second-degree AVB persisting at six hours; (4) third-degree AVB at any time during the observation period; (5) need for pharmacological intervention during the observation period. If a patient requires pharmacological intervention during the observation period after the first dose, monitoring should be conducted overnight in a medical facility, and should be repeated after the second dose of fingolimod.
Fingolimod is not recommended for patients susceptible to the development of heart rhythm disorders or significant bradycardia. These patients are those who: (1) have an increased risk of significant arrhythmias, namely those with Mobitz type II AVB, third-degree AVB, sinus node disease, sinoatrial block, corrected QT interval >450 ms (>470 ms if female), history of symptomatic bradycardia or recurrent syncope; (2) poorly tolerate significant bradycardia, namely individuals with ischemic heart disease, cerebrovascular disease, heart failure, poorly controlled hypertension, untreated severe sleep apnea, or history of myocardial infarction or cardiac arrest; (3) are undergoing treatment with class Ia or class III antiarrhythmic drugs; (4) are undergoing treatment with HR-reducing drugs, including beta-blockers and non-dihydropyridine calcium channel blockers. However, fingolimod is not contraindicated in these situations and can be administered if the anticipated benefit clearly outweighs the associated risk. In these cases, support from a cardiologist is recommended, in order to ascertain the best monitoring method and, when applicable or possible, modify concomitant therapy (for two to four weeks, temporary reduction of bradycardic drugs dose or replacement by drugs that do not decrease HR). It is recommended that the monitoring period be extended to include at least the first night after the first dose in the conditions described in (1) and (2) above, and also for patients described in (4) above who cannot suspend treatment with HR-reducing drugs.
The effects on HR and on atrioventricular conduction may recur on re-introduction of fingolimod treatment, depending on the duration of the interruption and the time elapsed since treatment initiation. It is recommended that monitoring after the first dose be repeated when treatment is interrupted for one or more days during the first two weeks of treatment, more than seven days during the third and fourth week of treatment, or two weeks or more after one month of treatment.
ConclusionsThe cardiac effects induced by fingolimod are due to functional antagonism of S1PR1 present in the cardiovascular system. In phase III clinical trials, fingolimod caused a small number of cases of sinus bradycardia and delayed atrioventricular conduction, usually within six hours after the first dose, and mostly asymptomatic, transient, resolving spontaneously and, therefore, clinically benign. Fingolimod-associated bradycardia can be reversed by atropine or isoprenaline if significant symptoms develop. Fingolimod therapy results in a small increase in blood pressure, which reaches its maximum value at around six months and remains stable thereafter. The clinical significance of this side effect remains unknown.
Conflicts of interestCarlos Aguiar has received personal compensation for lecturing from Novartis. Sónia Baptista has received personal compensation from Novartis, Merck Serono, Teva, and Biogen. Ricardo Pacheco is an employee of Novartis Farma S.A.
The authors would like to thank Miguel Vaz Afonso for his assistance with preparing this paper.






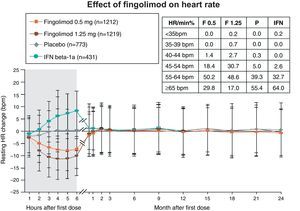 IFN beta-1a: interferon beta-1a. Adapted from DiMarco et al.46' title='Effects of fingolimod on heart rate: pooled analysis of the FREEDOMS, FREEDOMS II and TRANSFORMS studies. Results are expressed as mean ± standard deviation. The inserted table shows the minimum heart rate (HR) recorded in the first six hours after the first dose of fingolimod. F: fingolimod; IFN: interferon;
IFN beta-1a: interferon beta-1a. Adapted from DiMarco et al.46' title='Effects of fingolimod on heart rate: pooled analysis of the FREEDOMS, FREEDOMS II and TRANSFORMS studies. Results are expressed as mean ± standard deviation. The inserted table shows the minimum heart rate (HR) recorded in the first six hours after the first dose of fingolimod. F: fingolimod; IFN: interferon; 

