We aimed to assess the effects of successful ablation on impaired left ventricular global longitudinal strain (LV-GLS) in patients with frequent premature ventricular contractions (PVCs). We also evaluated the potential risk factors of impaired LV-GLS.
MethodsThirty-six consecutive patients without any structural heart disease, who were treated with radiofrequency (RF) ablation due to frequent PVCs, were included in the study. All patients were evaluated with standard transthoracic and two-dimensional speckle tracking echocardiography.
ResultsMean LV-GLS before ablation was 17.3±3.7 and 20.5±2.6 after ablation; the difference was statistically significant (p<0.01). Patients were categorized into two groups: those with LV-GLS value >−16% and those ≤16%. Low PVC E flow/post-PVC E flow and PVC SV/post-PVC SV ratios were associated with impaired LV-GLS.
ConclusionIn symptomatic patients with frequent PVCs and normal left ventricular ejection fraction, we observed significant improvement in LV-GLS value following successful RF ablation. Patients with impaired LV-GLS more often display non-ejecting PVCs and post-extrasystolic potentiation (PEP) compared to patients with normal LV-GLS.
O nosso objetivo foi avaliar os efeitos de ablação eficaz na deformação longitudinal global do ventrículo esquerdo (LV-GLS) em doentes com extrassístoles ventriculares (PVCs). Também avaliamos os potenciais fatores de risco de LVGLS anormal.
MétodosTrinta e seis doentes consecutivos sem doença cardíaca estrutural tratados com ablação por radiofrequência (RF) devido a PVCs frequentes foram incluídos no estudo. Todos os doentes foram avaliados com ecocardiografia transtorácica com speckle tracking bidimensional (2D-STE).
ResultadosOs valores médios de LVGLS antes da ablação foram de 17,3±3,7. Esse valor foi observado como 20,5±2,6 após a ablação e a diferença foi estatisticamente significativa (p<0,01). Os doentes foram classificados em dois grupos, LV-GLS >−16% e ≤16%. Baixo valor de PVC E flow/post-PVC E flow e PVC SV/post-PVC SV associaram-se com LV-GLS anormal.
ConclusãoEm doentes sintomáticos com PVCs frequentes e fração de ejeção do ventrículo esquerdo (FEVE) normal, observamos melhoria significativa no valor LV-GLS após ablação por RF bem-sucedida. Doentes com LV-GLS anormal apresentam frequentemente nonejecting PVCs e potencialização pós-extrassistólica (PEP) em comparação com doentes com LV-GLS normal.
Frequent premature ventricular contractions (PVCs) cause left ventricular (LV) dysfunction, and may even result in a form of cardiomyopathy known as premature ventricular contraction-induced cardiomyopathy (PVC-CM).1,2 The incidence of PVC-CM is approximately 7%, but this is likely to be a significant underestimation, since it was also reported to be as high as 30% in patients referred for catheter ablation.3,4 Although radiofrequency (RF) ablation and antiarrhythmic drugs are accepted strategies to reverse LV dysfunction due to PVCs,5,6 the minimum burden, origin (LV, RV, outflow, endocardial, epicardial), duration and coupling interval of PVCs required to impair LV function are unknown.7,8
In daily practice, PVC-CM is determined by the measurement of left ventricular ejection fraction (LVEF) using two-dimensional (2D) transthoracic echocardiography (TTE). Measuring LVEF enables detection of cardiomyopathy only in later stages, therefore, attempts have been made to find new imaging methods to detect PVC-CM at an earlier stage. Frequent PVCs may cause the development of cardiomyopathy, if only a significant portion of the myocardium has been affected over time.7,8 Myocardial strain can identify early stages of PVC-CM especially in patients with preserved LVEF.9 Therefore, strain imaging has emerged as a critical adjunct in the assessment of systolic function, particularly in patients whose LVEF is expected to worsen in the future.10
ObjectiveThe present study evaluated subtle and early forms of PVC-induced ventricular impairment in patients without any structural heart disease by using global longitudinal strain, which might be useful for early detection and risk stratification of cardiac dysfunction. In this study, we also evaluated changes in LV-GLS values after ablation as an indicator of regression in left ventricular dysfunction and various parameters showing the hemodynamic results of PVCs.
MethodsStudy populationThirty-six consecutive patients referred for radiofrequency (RF) ablation of frequent PVCs were included in the study. All patients underwent standard 12-lead electrocardiogram (ECG), 24-h Holter, and echocardiography. All patients were symptomatic and had at least one PVC-related symptom such as palpitation, lack of pulse, shortness of breath, weakness, or syncope. The research protocol was approved by the appointed local ethics committee. The following were excluded from the study: patients who had documented structural heart disease (transthoracic echocardiography and/or cardiac MRI) due to prior infarction or known dilated, arrhythmogenic, or valvular cardiomyopathy, and previous history of PVC ablation; patients in atrial fibrillation and pacemaker rhythm; those presenting with polymorphic PVCs and frequent non-sustained ventricular tachycardia on standard 12-lead ECG or 24-hour Holter recording.
A standard 12-lead ECG was recorded at 25 mm/s and 10 mm/mv for all patients. Premature ventricular complexes were defined as premature beats with abnormally shaped and prolonged QRS complex arising from an ectopic focus within ventricles. 24-Hour Holter recording (GE SEER, General Electric, USA) was performed before the procedure in every patient and PVC burden was noted. The percentage of PVCs was calculated by dividing the total number of PVCs by the total number of beats recorded during Holter monitoring.
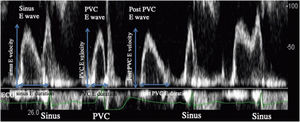
Echocardiographic assessments and strain analysisEchocardiographic examinations were performed by expert cardiologists who were unaware of patients’ other data, by using iE33 (Philips Medical Systems, Andover, Mass). All echocardiographic parameters such as left ventricular diastolic dimension (LVDd), LVEF, E wave, A wave velocity, E/A, E′ and A′ were assessed in accordance with the recommendations of the American Society of Echocardiography.11 LVEF was calculated by using the Simpson's method and to avoid post-extra systolic potentiation effects on LVEF, second consecutive sinus beat was evaluated. In all patients, LV inflow and LV outflow patterns were evaluated at the time of PVC, post-extra systolic sinus beat and continuous sinus beats. The maximum velocities and LV inflow wave durations of following continuous sinus beats were measured in the LV diastolic phase, the multiplication of maximum velocity and LV inflow wave duration was defined as “sinus E wave flow”. The E wave at the time of PVC was defined as “PVC E wave”, the multiplication of maximum velocity and PVC E wave duration was defined as “PVC E wave flow”. Post-extra systolic E wave was defined as “post-PVC E wave”, the multiplication of maximum velocity and post-PVC E wave duration was defined as “post-PVC E wave flow” (Figure 1). Stroke volumes (SV) were measured using the velocity time integrals at continuous sinus beats, at PVC and at post-extra systolic beat. It was then defined as “sinus SV”, “PVC SV” and “post-PVC SV”, respectively.12
Left ventricular (LV) inflow on echocardiography. During the sinus diastolic phase, the initial LV inflow was defined as “sinus E wave”, and the value obtained by multiplying the maximum sinus E wave velocity and duration was defined as the “sinus E wave flow”. When PVC occurred in the middle of the E wave in the sinus diastolic phase, this E wave was defined as the “PVC E wave”. During the PVC diastolic phase, LV inflow was defined as “post-PVC E wave”. These parameters were calculated as: sinus E wave flow=(sinus E velocity)×(sinus E duration); PVC E wave flow=(PVC E velocity)×(PVC E duration); post-PVC E wave flow=(post-PVC E velocity)×(post-PVC E duration). PVC: premature ventricular contraction.
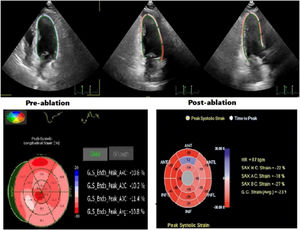
Strain measurements were performed using custom software (MVQ, QLAB, Philips). Digital cineloops of apical two-, three-, four-chamber and parasternal short-axis images at basal, midventricular and apical levels were recorded from the peak of the R wave at the end of expiration at a frame rate of 50–90 frames/sec. They were stored on optical discs in Digital Imaging and Communications in Medicine format for later offline analysis. The averages of three cardiac cycles were used in the analysis. For each heartbeat, the duration between the start of the QRS complex to the start of the next QRS complex was defined as a cardiac cycle. Wide QRS complex without a preceding P-wave was defined as PVC. The reference point was placed at the beginning of the PVC QRS complex and ended at the beginning of the next sinus rhythm QRS complex. For each of the short-axis views, the sampling points were placed manually along the endocardium at LV base, middle and apex, and for apical 2-, 3-, and 4-chamber views, three sampling points were placed manually at septal mitral annulus, lateral corner and apical endocardium at end-diastole. The software tracked endocardial contours automatically and generated a region of interest. The quality of myocardial tracking was checked visually, and the process was repeated or manually corrected if unsatisfactory tracking was obtained. The graphics of deformation parameters of each segment were then automatically formed, and the average peak strain values were obtained. The global longitudinal strain (GLS) was assessed as the average of the segmental value (Figure 2). Since the normal LV-GLS value ranges from −15.9% to −22.1% in previous studies, normal LV-GLS was accepted as ≤−16%, based on the current literature.13 Echocardiographic examinations and Q-LAB evaluations of strain images were performed by cardiologists experienced in STE. Experts were defined as those who had publications on this subject and at least 10 years’ echocardiography experience and who perform >500 echocardiography procedures per year. Cohen's kappa co-efficient that evaluates interobserver and intraobserver variability was >90% for all ECG and echocardiography measurements.
Ablation procedureAntiarrhythmic drugs were discontinued at least five half-lives before the procedure. Isoproterenol was given when PVCs were absent at the beginning of the procedure. Activation mapping and pace mapping was used in combination. RF energy was delivered with irrigated-tip catheters using power of 30–50 W. Acute success was defined by elimination of the targeted PVC after 30 minutes of observation with and without isoproterenol infusion. Patients who did not meet the acute success criteria were excluded from the study.
Follow-upPatients were categorized into two groups in the form of those with LV-LGS >−16% (Group 1, 16 patients) and those ≤−16% (Group 2, 20 patients). Control echocardiography was performed in all patients within three to six months after the procedure. 24-Hour Holter recording was performed in each patient after ablation. A >80% decrease in PVC burden compared to baseline was defined as long-term success of the ablation procedure.
Statistical methodsCategorical variables are expressed as numbers and percentages and were compared using X2 test or the Fisher exact test as appropriate. Continuous variables were expressed as mean±SD and were compared using unpaired T test or analysis of variance for comparison between several groups. Paired sample T test was used to compare the data before and after ablation in each group. Correlations between numerical values were assessed by nonparametric Spearman test. All statistical studies were carried out using the Statistical Package for Social Sciences software (SPSS software for Windows, version 23.0. IBM Corp. Armonk, NY, USA).
ResultsThirty-six symptomatic patients (16 females, age 50.3±9.7 years) were enrolled into the study. All patients used at least one antiarrhythmic drug before ablation (beta-blocker, calcium channel blocker, amiodarone, propafenone, sotalol). In the 24-hour outpatient Holter recordings obtained after the ablation procedure, it was observed that success (>80% decrease of PVC burden compared to baseline) was achieved in all patients.
The mean LV-GLS value before ablation was 17.3±3.7. This value was observed as 20.5±2.6 after ablation and the difference was statistically significant (p<0.01). Based on the existing literature17 where normal LV-GLS ranged from −15.9% to −22.1%, patients were categorized into two groups in the form of those having LV-LGS value >−16% and ≤−16%. In Group 1, whose LV-GLS values were >−16%, it was observed that the mean LV-GLS value improved significantly after ablation (−12.6±2.0 vs. −18.3±1.9, p<0.01). Although there was a trend toward improvement after ablation in Group 2, whose LV-GLS values were within the normal range, this trend did not reach statistical significance (18.9±2.1 vs. 20.6±1.8, p=0.06). After ablation, there were no significant changes in any of the common transthoracic echocardiographic parameters listed in Table 3. There were no significant differences in age, sex, body mass index, serum creatinine levels, hematocrit levels, and the prevalence of major comorbidities including diabetes, hypertension and smoking between groups. Although PVC history in years was longer in Group 1, the difference did not reach statistical significance (p=0.054) (Table 1).
Baseline characteristics.
| Group 1 (n: 16) | Group 2 (n: 20) | p | |
|---|---|---|---|
| Age (years) | 50.63±9.98 | 50.05±9.87 | 0.872 |
| Male, n (%) | 9 (56.2%) | 11 (55.0%) | 0.940 |
| Body mass index (kg/m2) | 24.11±2.56 | 24.03±2.75 | 0.830 |
| Hypertension, n (%) | 7 (43.7%) | 9 (45.0%) | 0.940 |
| Diabetes, n (%) | 3 (18.7%) | 4 (20.0%) | 0.925 |
| Smokers, n (%) | 4 (25.0%) | 5 (25.0%) | 1.000 |
| Hematocrit (%) | 38.87±1.99 | 39.73±2.76 | 0.174 |
| Serum creatinine (mg/dL) | 0.92±0.21 | 0.93±0.23 | 0.462 |
| PVC history (years) | 3.50±2.03 | 2.45±1.96 | 0.054 |
| Symptoms, n (%) | 16 (100%) | 20 (100%) | 1.000 |
| Antiarrhythmic drug before ablation, n (%) | 16 (100%) | 20 (100%) | 1.000 |
LVGLS: left ventricular global longitudinal strain; PVC: premature ventricular contraction.
Group 1: Patients with LVGLS >−16%.
Group 2: Patients with LVGLS ≤−16%.
The sites of PVC origins in patients who underwent successful ablation were as follows: right ventricular outflow tract (RVOT) 19 (52.7%), left ventricular outflow tract (LVOT) 9 (25%), Parahisian 2 (5.5%), papillary muscles 2 (5.5%), aortomitral continuity (AMC) 2 (5.5%), mitral valve 1 (2.7%), tricuspid valve 1 (2.7%). Mean procedure time was 138±53 minutes, fluoroscopy time was 19±34 minutes, and RF ablation time was 7±5 minutes (p=NS, between groups).
Comparison of ECG and 24-hour Holter recordings data between groups showed that PVCs coupling interval time was found to be significantly longer in Group 1 (Table 2). Total PVCs and PVCs burden were higher in Group 1 but the difference was not statistically significant. Other parameters were also found to be similar between groups (Table 2).
Various electrocardiographic and Holter monitoring parameters of the study patients.
| Group 1 (n: 16) | Group 2 (n: 20) | p | |
|---|---|---|---|
| Mean heart rate | 71.42±3.64 | 74.61±7.18 | 0.571 |
| PVC QRS duration (ms) | 141±42 | 136±53 | 0.349 |
| PVC QRS amplitude (mV) | 2.05±0.6 | 1.9±0.6 | 0.284 |
| PVCs QRS interval time (ms) | 151±21 | 146±19 | 0.098 |
| PVCs coupling interval time (ms) | 478±108 | 445±121 | 0.027 |
| Total PVCs (n) | 16956-7061 | 14015-5360 | 0.089 |
| PVCs burden (%) | 20-9 | 17-6 | 0.075 |
LVGLS: left ventricular global longitudinal strain; PVC: premature ventricular contraction.
The values are shown as mean±SD, median-interquartile range, or n (%).
Group 1: Patients with LVGLS >−16%.
Group 2: Patients with LVGLS ≤−16%.
LVDd, LVEF, left atrial diameter, E/A ratio, E′ wave, E/E′ in Group 1 did not differ from Group 2 (Table 3). All patients with LV hypertrophy were excluded, so there was no difference in LV mass index between the groups. No patients had moderate or severe mitral regurgitation. While there was no difference in sinus E wave flow between groups (20.0±2.8 vs. 20.3±3.2, p=0.55), PVC E wave flow was lower in Group 1 than in Group 2 (7.6±2.8 vs. 13.6±3.4, p=0.02). Post-PVC E wave flow in Group 1 was higher in comparison to Group 2 (27.7±4.3 vs. 19.8±3.5, p=0.04). Similarly, while there was no difference in sinus SV between groups (74.2±7.7 vs. 77.7±10.7, p=0.12), PVC SV was lower in Group 1 than in Group 2 (17.6±5.8 vs. 35.2±6.9, p=0.02), and post PVC SV was higher in Group 1 in comparison to Group 2 (94.9±4.6 vs. 79.1±8.2, p=0.03). E wave flow and SV values at the time of PVC and post-extra systolic sinus beat were proportioned between the two groups. PVC E flow/post-PVC E flow and PVC SV/post-PVC SV were both significantly lower in Group 1 than in Group 2 (Table 3).
Echocardiographic findings of the study patients.
| Group 1 (n: 16) | Group 2 (n: 20) | P | |
|---|---|---|---|
| LVDd (mm) | 51.0±3.3 | 49.1±3.7 | 0.74 |
| LVEF (%) | 62.1±3.2 | 64.7±3.8 | 0.38 |
| LA diameter (mm) | 39.3±2.0 | 38.1±1.6 | 0.38 |
| LV mass index (g/m2) | 80.2±6.4 | 78.1±6.8 | 0.34 |
| E/A ratio | 1.0±0.4 | 1.2±0.4 | 0.97 |
| E/E′ ratio | 12.2±1.0 | 10.2±0.8 | 0.11 |
| Sinus E wave flow (cm) | 20.0±2.9 | 20.3±3.2 | 0.55 |
| PVC E wave flow (cm) | 7.6±2.8 | 13.6±3.4 | 0.02 |
| Post-PVC E wave flow (cm) | 27.7±4.3 | 19.8±3.5 | 0.04 |
| Sinus SV (mL) | 74.2±7.7 | 77.7±10.7 | 0.12 |
| PVC SV (mL) | 17.6±5.8 | 35.2±6.9 | 0.02 |
| Post-PVC SV (mL) | 94.9±4.6 | 79.1±8.2 | 0.03 |
| PVC SV/post-PVC SV | 0.2±0.02 | 0.6±0.06 | <0.01 |
| PVC E flow/post-PVC E flow | 0.3±0.03 | 0.7±0.11 | <0.01 |
LA: left atrial; LVDd: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVGLS: left ventricular global longitudinal strain; PVC: premature ventricular contraction; SV: stroke volume.
Group 1: Patients with LVGLS >−16%.
Group 2: Patients with LVGLS ≤−16%.
Although PVCs used to be considered as mostly benign, long-term frequent PVCs may lead to PVC-CM in some patients,13 via pathogenic mechanisms possibly involving ventricular systolic dyssynchrony during PVCs, longer coupling intervals, and post-extrasystolic potentiation.14–17
PVC-CM was first classified as an indication of catheter ablation by European Heart Rhythm Association/Heart Rhythm Society Expert Consensus on Catheter Ablation of Ventricular Arrhythmias in 2009.18 However, there is uncertainty about the exact diagnostic criteria for recognizing PVC-CM, so the diagnosis is mostly made retrospectively and by exclusion. It also remains controversial whether intervention is necessary in patients with frequent PVCs who are asymptomatic or have normal LVEF. In this study, we used 2D-STE and evaluated strain values to detect cardiac dysfunction in patients with frequent PVCs in the absence of concomitant structural heart disease. It should be remembered that there are varying degrees of cardiac dysfunction even in patients without abnormal findings on conventional echocardiography. These patients present with reduced global and regional strain values, uneven overall color of bull's-eye plots, disordered strain, fewer smooth curves, and significantly reduced wave amplitudes.
Myocardial strain can identify early stages of PVC-CM particularly in patients with preserved LVEF. Therefore, strain imaging has emerged as a critical adjunct in the assessment of systolic function, especially in patients whose LV function is expected to deteriorate progressively.19
Recent studies reported that LV-GLS values were significantly decreased in patients with frequent PVCs and preserved LVEF.19–21 Studies evaluating the effects of RF ablation on LV-GLS in patients with preserved LVEF are limited. In a recent study, Koca et al. reported that RF ablation increased LV longitudinal strain values.22 Uhm et al. showed the positive effect of RF ablation on RV-GLS in patients with frequent PVCs originating from RVOT.23 A recent article also demonstrated impaired LV-GLS in the sinus rhythm beat preceding PVC. This finding suggests that disturbances in cellular physiological processes such as excitation–contraction coupling (autonomic or calcium handling) may play a role in the generation of frequent PVCs.24
In our study, similar to the result of the abovementioned study, we found that there was a significant increase in LV-GLS after RF ablation in patients with normal LVEF. This improvement was statistically significant in Group 1 patients whose LV-GLS was >−16 before ablation. In Group 2 patients, whose LV-GLS values were within the normal range, there was a slight trend toward improvement, especially in patients whose values were close to the reference; it did not, however, achieve statistical significance. A larger study sample is needed to determine the exact reference values.
In this study our aim was to evaluate differences between patients according to their LV-GLS demonstrated by the 2D-STE before ablation. As a result, there were no remarkable differences in the clinical, electrocardiographic, and common echocardiographic parameters between the two groups. On the other hand, the group with impaired LV-GLS values (Group 1) had significantly lower PVC E wave flow and PVC SV with higher post-PVC E wave flow and post-PVC SV values. As a result, PVC E flow/post-PVC E flow and PVC SV/post-PVC SV rates were significantly lower in Group 1 than in Group 2.
The mechanism of PVC-CM is not clear. There is general agreement that the higher the number of PVC per day, the greater the risk of developing cardiomyopathy.25 In our study, the number of PVCs per day was higher in Group 1, but the difference was not statistically significant (p=0.089). Besides PVC burden, some other risks factors for progression to cardiomyopathy have been identified, but there are inconsistencies in studies. Patient characteristics such as increasing age,26 high body mass index,27 and male gender28 were found to be related to PVC-CM. In our study, there were no significant difference in patient characteristics between the groups. Longer exposure to frequent PVCs increases the risk of subsequent cardiomyopathy.4 Although Group 1 had a longer history of PVC in our study, the difference did not reach statistical significance (p=0.054). Some electrocardiogram (ECG) characteristics (more than one PVC morphology, presence of non-sustained VT) may promote cardiomyopathy.3 In our study, patients with this ECG characteristics were excluded from the study. Another indicator of PVC-induced cardiomyopathy is a greater QRS width of the PVCs.3 In our study, there was no difference in PVC width between the groups. Longer coupling interval,15 and presence of interpolation29 were associated with PVC-induced cardiomyopathy due to mechanisms such as dyssynchrony and atrioventricular dissociation. Similarly, we found that the PVC coupling interval time was higher in Group 1 patients.
Some PVCs do not create sufficient ejection volume or pressure for aortic valve opening and detectable aortic pressure (mechanical systole), leading to a concealed mechanical bradycardia. In our study, PVCs in Group 2 were more efficient in ejecting into the aorta than PVCs in Group 1, resulting in less concealed mechanical bradycardia. Frequent mechanically inefficient PVCs lead to sustained concealed mechanical bradycardia, decreased cardiac output30 and increased LV diastolic pressures, volume overload, LV dilation, and LV systolic dysfunction. Furthermore, repeated lack of arterial pulse can be responsible for neurovegetative imbalance through baroreceptor reflex mechanism,31,32 which may worsen myocardial function over time. In our study, patients with impaired LV-GLS (Group 1) had significantly lower PVC E wave flow and PVC SV values, which supports the abovementioned mechanisms.
Some authors consider post-extrasystolic potentiation (PEP) as a mechanism for PVC-CM. PEP significantly increases myocardial oxygen consumption,33,34 and when frequent this increased energy consumption may cause systolic dysfunction. However, this hypothesis has never been investigated. In our study, patients with impaired LV-GLS had a significantly higher post-PVC E wave flow and post-PVC SV values. Although we indicated a significant correlation between poor hemodynamic performance of PVCs and the presence of impaired LV-GLS, it is difficult to conclude from the results of this study that inefficient PVCs cause cardiomyopathy. Definitive conclusions cannot be drawn from this study, and only prospective studies can resolve this issue.
LimitationsInterpretation of the findings of this study is limited due to several factors. The sample size was limited, which precluded the definition of PVC-induced cardiomyopathy predictors and strain value cutoffs to determine the need for intervention including radiofrequency ablation. We were unable to examine variables that would have been useful in identifying subclinical left ventricular dysfunction in patients with normal ejection fraction, such as brain natriuretic peptide levels, the Kansas City Cardiomyopathy Questionnaire, and the six-minute walk test. Long-term follow-up is needed to check the results of the present study and to assess cardiac function recovery in patients with frequent PVCs undergoing clinical intervention. 2D-STE requires high quality images, comparison of experimental results is restricted because parameters vary between different echocardiography devices and software workstations used for analysis. However, interobserver and intraobserver variability in strain values was good and consistent with previous studies. Future studies conducted with large sample size are required to determine the exact reference values.
ConclusionIn symptomatic patients with frequent PVCs and normal LVEF, we observed significant improvement in LV-GLS following successful ablation. Patients with impaired LV-GLS more often displayed non-ejecting PVCs and PEP compared to patients with normal LV-GLS. Lower PVC E wave flow, PVC SV and higher post-PVC E wave flow, post-PVC SV values were observed in patients with impaired LV-GLS. Further studies are required to determine these associations.
Author contributionsAll authors contributed to: (1) conception and design, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be published.
Ethical approvalThe study was performed in compliance with the principles outlined in the Declaration of Helsinki and approved by the ethics committee of TC. Atlas University.
FundingThe authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interestThe authors have no conflicts of interest to declare.