Recurrence of cardiac myxoma is a rare condition, observed in about 3% of patients in sporadic cases, although it is more frequent in familial ones.
Several mechanisms have been proposed to explain such recurrence, and the importance of increased vascularization as a facilitating feature is the subject of debate.
The authors report the case of a non-familial right atrial myxoma, unusual for both its histopathology and recurrence.
A recorrência de mixomas cardíacos é rara, acontecendo em cerca de 3% dos doentes com mixomas esporádicos, embora seja mais frequente nos casos familiares.
Vários mecanismos foram propostos para explicar as recorrências e a importância da vascularização aumentada como fenómeno facilitador é controversa.
Os autores descrevem o caso de um mixoma esporádico do coração direito, peculiar pelas suas características histológicas e recorrência.
Myxomas are the most common primary cardiac tumors, representing 30–50% of cardiac neoplastic lesions identified in anatomopathological series. Although preferentially located in the left atrium, myxoma may also appear in the right atrium (up to 20% of cases), ventricular chambers and, rarely, on cardiac valves.1,2
Its clinical behavior may be “malignant” with embolic events, valvular dysfunction and hemodynamic compromise.3 Nevertheless these benign tumors are usually cured by surgery and synchronous and/or recurrent forms are rarely described, mainly in the context of familial syndromes.
Case reportWe report the case of a 38-year-old man referred to our institution for recurrence of a heart tumor. His history was negative for other cardiovascular problems or family history of heart disease and tumors. There was no evidence of Carney complex.
Three years previously he had undergone surgical removal of a large right atrial myxoma, detected on a transthoracic echocardiogram ordered because of fatigue, malaise, shortness of breath and chest tightness. The mass was described as fully occupying the right atrial cavity (68×53 mm in apical view), extending towards the opening of the inferior vena cava and prolapsing into the right ventricle, causing functional tricuspid valve stenosis. Transesophageal study located the stalk in the atrial septum.
Surgical excision was reported as complete, requiring extensive excision and reconstruction of the atrial septum with autologous pericardium.
Histopathology confirmed the diagnosis of a completely resected right atrial myxoma with free tumor margins. Follow-up transthoracic echocardiography after 12 months showed no right atrial mass.
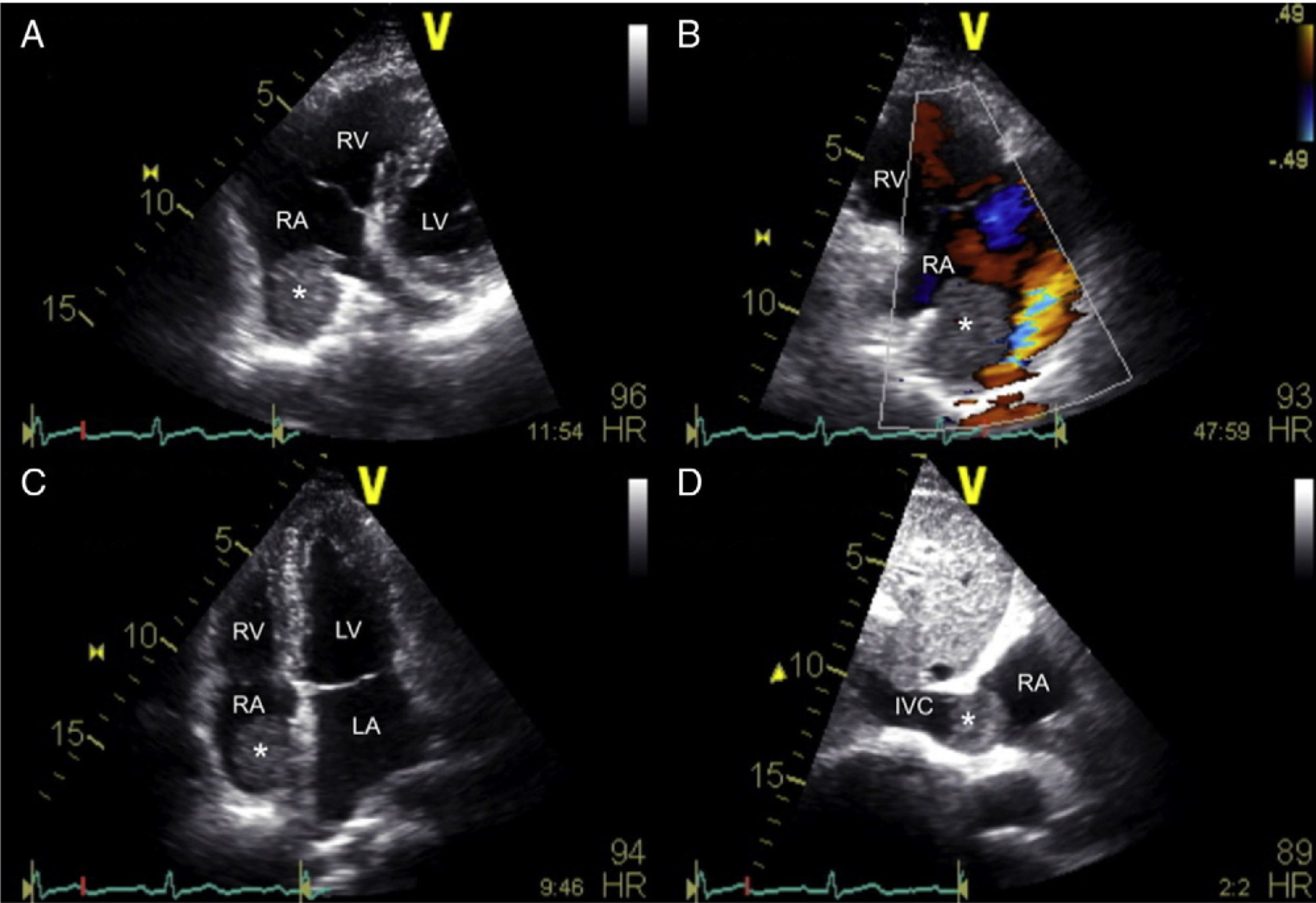
Two years later, a redo routine echocardiographic evaluation revealed the presence of a new small spherical non-mobile right atrial mass (26×26 mm). Its echocardiographic appearance was soft, smooth, and homogeneous. It was located in a small recess near the inferior vena cava opening. Impaired venous return was suspected due to the absence of inferior vena cava respiratory variation, and flow acceleration at the confluence with the right atrium (Figure 1). Transesophageal echocardiography confirmed these findings and ruled out the presence of other tumor foci. Both the mass and the residual atrial septum were shown by Doppler imaging to be richly vascularized (Figure 2).
Transthoracic echocardiography study showing a right atrial mass located near the inferior vena cava opening (A and B – non-conventional views, C – apical 4-chamber view, D – subcostal view) and flow acceleration at the confluence with the right atrium (B). *: right atrial mass; IVC: inferior vena cava; LA: left atrium; LV: left ventricle; RA: right atrium; RV: right ventricle.
The patient underwent excision of the mass, which required venous and atrial septum reconstruction as a result of the broad base of tumor implantation.
Histopathological diagnosis was consistent with benign cardiac myxoma (Figure 3A). A peculiar morphological aspect attracted the pathologist's attention: an increased number of vessels randomly located around the insertion area, with irregular contours, thick walls, and fibrointimal hyperplasia (Figure 3B). At this point, a review of the slides from the first cardiac myxoma showed similar morphological features.
Twelve months after the second operation, the patient remains asymptomatic, without abnormal findings on follow-up transthoracic echocardiography.
DiscussionThe estimated recurrence rate of sporadic myxoma is 2–3%.4–6 They usually appear during the first four years, although they can emerge within a few months to several years after surgical excision.7–9 Unlike primary myxoma, which is more frequent in women, tumor recurrence appears to be more frequent in men.10
The first case of myxoma recurrence was described by Gerbode et al. in 1967, several years after the first surgical removal of such tumors.11 At that time, incomplete surgical resection was presumed to be the cause. Since then, several cases of tumor recurrence have been reported, not only after extensive excisions but also arising on the opposite side of the heart from the initial one.12
Four different possible mechanisms have been put forward to explain recurrence: inadequate resection, totipotent multicentricity, inheritance (familial type), and metastatic recurrence.1,5,12,13
There is some histopathological evidence of a tendency for increasing aggressiveness and possible malignant degeneration into forms of myxosarcoma.1,10
Even though the mechanism of recurrence is difficult to confirm in an individual case, the possibility of a familial syndrome should always be considered. The presence of concurrent cardiac lesions and clinical features suggestive of syndromic association (such as hyperpigmentation, endocrine disease, or benign extracardiac connective tissue tumors) should raise the suspicion of familial transmission,14 for which causative mutations have been identified.15
The unusual nature of this case lies in a less common location of the tumor (in the right atrium), and in its recurrence, considering this is a case of the sporadic form of the disease. Moreover, both echocardiography and histology provided evidence of marked vascularization, a feature seldom encountered in myxomas, but usually present in malignant lesions. In fact, the presence of these large vessels in the tumor base, pedicle, and surrounding structures could facilitate development and/or recurrence of a neoplastic mass, acting as stimuli for excessive growth and differentiation. A strong angiogenic potential driven by the release of VEGF by macrophages and the tumor cells, as well as autocrine activation of VEGF receptors on these cells, has been shown in cardiac myxoma, thereby perpetuating the angiogenic response within these tumors.16 Abnormal blood supply might thus be an explanation for the oncogenic-like behavior of otherwise benign quiescent multicentric totipotent cells.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.