Iron deficiency (IDef) is a prevalent condition in patients with heart disease and in those with heart failure (HF). Evidence has shown that this deficit is associated with worse prognosis. Data in literature are scarce on the prognostic impact of IDef in acute coronary syndromes (ACS), which is the main objective of this study.
MethodsObservational, retrospective study which included 817 patients admitted for ACS. Two groups were defined according to the presence (n=298) or absence of IDef (n=519) on admission. The clinical event under study was the occurrence of death or severe HF in the long term. Independent predictors of prognosis were determined with logistic regression analysis.
ResultsThirty-six percent of patients had IDef. There was higher mortality (p=0.004), higher incidence of HF (p=0.011) during follow-up and a higher rate of hospital readmissions (p=0.048) in this group. IDef was an independent predictor of death or severe HF in follow-up, along with anemia, left ventricular dysfunction, renal dysfunction and the absence of revascularization. IDef also enabled us to further stratify the prognosis of patients without anemia based on the occurrence of death or severe HF and those with lower Killip classes (≤2) based on the occurrence of death.
ConclusionIDef was an independent predictor of death or severe HF in patients admitted with ACS and enabled additional stratification for those without anemia on admission and in those with Killip classes ≤2.
O défice de ferro (DeF) é uma condição prevalente em doentes com patologia cardíaca e naqueles com Insuficiência Cardíaca (IC) demonstrou-se que esse défice se associa a pior prognóstico. Existem poucos dados na literatura relativamente ao impacto prognóstico do DeF nas Síndromes Coronárias Agudas (SCA), sendo este o principal objetivo deste estudo.
MétodosEstudo observacional retrospetivo que incluiu 817 doentes admitidos por SCA. Definiram-se dois grupos de acordo com a presença (n=298) ou ausência de DeF (n=519) à admissão. O evento clínico estudado foi a ocorrência de morte ou IC grave a longo prazo. Calcularam-se os preditores independentes de prognóstico com base na análise de regressão logística.
ResultadosVerificou-se que 36% dos doentes tinham DeF. Estes doentes apresentaram maior taxa de mortalidade (p=0,004) e de IC (p=0,011) durante o follow-up bem como maior taxa de readmissões hospitalares (p=0,048).
O DeF foi preditor independente de morte ou IC grave no follow-up, a par da anemia, da disfunção do ventrículo esquerdo, da disfunção renal e da ausência de revascularização.
Além disso, o DeF permitiu estratificar adicionalmente o prognóstico dos doentes sem anemia em termos de ocorrência de morte ou IC grave e aqueles com classes de Killip mais baixas (≤2) em termos da ocorrência de morte.
ConclusãoO DeF foi um factor preditor independente de morte ou IC grave nos doentes admitidos com SCA e permitiu estratificar adicionalmente aqueles sem anemia ou com classes de Killip ≤ 2 à admissão.
Iron is an essential micronutrient in multiple cellular functions. In addition to its critical role in erythropoiesis, it is also involved in oxygen transport, in the immune cell response, and is particularly important in mitochondria, where it catalyzes enzymatic reactions and regulates oxidative metabolism.1–6 The homeostasis of iron metabolism is dependent on several mechanisms and iron is obtained from two main sources: diet and recycling of senescent erythrocytes.7
Dietary iron is absorbed in the jejunum via a specific transporter.1,3,8 When the body needs iron, it is released into circulation through ferroportin, binding to transferrin so that it can be transported in plasma.1,3,8 Unused iron is stored in the liver, spleen and bone marrow in the form of ferritin.1,3,8
Ferritin is involved in several physiological and pathological cellular processes and is often used as a serum marker of iron stores. It plays an important role in both the diagnosis and follow-up of IDef.9–12
Hepcidin is a hormonal regulator of iron homeostasis; it inhibits ferroportin, thus decreasing gastrointestinal absorption of iron and, as a consequence, it is no longer released from macrophages and hepatocytes. This then leads to a decrease in iron concentration in the blood and in its availability for cell metabolism.1,5,7,11,13
Iron deficiency is a common condition and can be classified as absolute or functional.1,5,13 Absolute IDef is caused by depleted iron stores, possible causes being insufficient iron intake, gastrointestinal malabsorption and/or hemorrhage, resulting in ferritin levels <100 ug/L.1,8,13 Functional IDef results from decreased iron bioavailability compared to demand, despite reserves having adequate levels, due to the increased production of hepcidin.1,5,8,13 It usually results in ferritin levels of 100–299 ug/L and transferrin saturation (TSAT) <20%.1,8 Anemia can result from depleted iron reserves, however, in numerous situations, despite the decrease in these reserves, hemoglobin values are at levels considered normal.3 Usually, anaemia stablishes when there is an important decrease in iron reserves.3
Particular importance was given to adequate levels of iron when it was found that both a deficit and excess of iron alter intracellular functions, especially in the mitochondria, leading to increased oxidative stress and deranged energy metabolism.4,8,11
Cells with high energy requirements, such as cardiomyocytes, have additional signaling pathways compared to storage cells, thus increasing iron reabsorption and decreasing extracellular flux in the case of iron deficiency.1,3,4
The pathophysiology associated with cardiac remodeling in IDef is not yet well-defined. However, in mice with IDef, left ventricular (LV) dimensions are increased associated with mitochondrial changes and sarcomere organisation.14,15 In addition, anemia with IDef causes cardiac hypoxia and fibrosis, factors that also contribute to cardiac dysfunction.14
Iron metabolism disorders are common in patients with heart disease.4,16,17 Of these, the most widely substantiated is chronic heart failure (CHF) and, in these patients, the most common cause of anemia is IDef.14,16,18,19 According to current recommendations, these patients should undergo testing that includes serum iron, serum transferrin, TSAT and ferritin, regardless of the presence or absence of anemia, since IDef may be present many years before anemia develops.4,5,20,21 The role of iron in this disease has been widely studied in recent years, demonstrating that the greater the IDef, the more common and more severe adverse cardiovascular events are.5,22
Intravenous iron supplementation is indicated in patients with CHF and reduced EF who have both absolute and functional IDef.5,18,21,23Although oral iron supplementation can be considered, especially if the condition is not acute, unstable or symptomatic, intravenous over oral supplementation has been demonstrated to be superior.5,6,22 This is mainly due to the interference of CHF inflammation in the absorption of oral iron (by altering the actions of hepcidin and ferroportin). It has been proven that patients with CHF and reduced EF – regardless of the presence of anemia – benefit from the administration of ferric carboxymaltose, showing improvement in New York Heart Association (NYHA) functional class and exercise capacity.2,8,18,21,23,24
Acute coronary syndromes (ACS) are among the most frequent causes of death worldwide.25 They are characterized by an acute clinical status compatible with myocardial ischemia, which may result in cardiomyocyte necrosis.26 They may present as: acute myocardial infarction with (STEMI) or without ST-segment elevation (NSTEMI) or unstable angina; all of them potentially fatal.27,28
Contrary to what has been found in CHF, data in the literature are scarce on the role of IDef in ACS. Given its prevalence and associated morbidity and mortality, it is relevant to assess to what extent iron metabolism disorders have prognostic impact in these patients.16,29
The main objective of this study was to determine the prevalence and prognostic impact of IDef in patients with ACS by assessing the occurrence of death or development of severe HF. It also sought to assess whether IDef adds additional predictive power in the risk stratification of patients with ACS, regardless of the presence of anemia.
MethodsAn observational longitudinal design study with retrospective data collection was conducted. It had a descriptive and an analytical component.
Study population and sampleThe study population included all patients admitted to a coronary unit with a diagnosis of ACS between July 2013 and June 2015.
Patients with chronic inflammatory disease, chronic liver disease, end-stage kidney disease (CKD) (stage V of the Kidney Disease Outcomes Initiative Classification), malignant tumors and/or on iron or erythropoietin supplementation were excluded from this study. These criteria were used because of the changes in ferritin and erythropoiesis caused by these conditions.15,30 Patients in whom the evaluation of the IDef was not possible due to lack of laboratory results of serum iron, ferritin and total iron fixation capacity levels were also excluded.
Data collectionPatients were identified by a casefile number, consisting of a numerical code; subsequently, a new code was attributed to them to ensure anonymity.
Clinical information collected included demographic and anthropometric data, personal history (especially cardiovascular risk factors [CVRF]), previous cardiovascular (CV) disease, previous heart surgery and/or interventional catheterization, kidney or liver disease, tumors, chronic inflammatory disease), usual medication (antiplatelets, hypocoagulants, antihypertensive, anti-dyslipidemic, antianginal), clinical evaluation (signs and symptoms), laboratory test data (hemogram, leucogram, biochemistry, inflammatory parameters, kidney function, iron kinetic parameters), other complementary diagnostic and therapeutic methods (cardiac catheterization and echocardiogram), treatment performed (therapy started and procedures performed), clinical evolution during hospitalization and during the follow-up period (especially cardiovascular events). No therapeutic assessment was performed during follow-up.
Blood was collected for determining serum iron, ferritin and total iron binding capacity (TIBC) levels in the first 24 hours of hospitalization. To obtain TSAT, the following formula was used: TSAT=serum iron/TIBC × 100.11,19,20,21
Iron deficiency was defined as ferritin <100 μg/l or ferritin >100 μg/l and <300 μg/l and TSAT<20%, accounting for both absolute and functional deficiency.5,23 Anemia was defined as hemoglobin <13 g/dl for men and <12 g/dl for women.3,13,17
The data obtained was recorded in a Microsoft EXCEL® table and subsequently entered and processed in IBM® Statistical Package for the Social Science (SPSS®) version 22 software.
Data analysisPatients who did not meet the exclusion criteria were included and divided into two groups according to the presence or absence of IDef.
Descriptive statistics were used to characterize the sample and compare the study groups. Categorical variables were expressed as percentages and continuous variables as mean ± standard deviation or median, maximum and minimum values.
To assess normality, the Kolmogorov–Smirnov test was applied and taking into account the number of patients in the sample and the number of patients in each group, the flatness coefficient and skewness were analyzed. In the case of flatness and skewness values close to 0, normal distribution was assumed.31
In order to compare the groups and assess the differences between them, the Student's t-test for independent samples (parametric test) or the Mann–Whitney test (non-parametric test) was used for continuous variables, after having checked whether the dependent variable presented a normal distribution or not, respectively. Cohen's d was used as a measure of effect size for the Student's t-test and r for the Mann–Whitney test. For Cohen's d, we considered a small effect for values close to 0.2, medium for 0.5 and large for 0.8. For r a small effect was attributed to values close to 0.1, medium for 0.3 and large for 0.5.
The chi-square test was used to compare categorical variables between the groups. As these were mainly dichotomous variables, 2×2 tables were obtained. Interpretation of the results depended on the assumption - percentage (%) of cells with n<5. Continuity correction values were reported for the 2×2 tables and Pearson Chi-Square for tables larger than 2×2. Phi coefficient was used as a measure of effect size) values close to 0.1 represent a small effect, 0.3 a medium effect and 0.5 a large effect.
Binary logistic regression was used to determine possible predictors of death or severe HF. All predictors included in the model showed a statistically significant relationship with death or severe HF in the univariate analysis. The number of variables inserted into the model also took into account the number of events occurred.
Kaplan–Meyer curves were used to estimate ACS patient survival and the log rank test to assess differences between groups.
It was further assessed whether IDef adds additional predictive power in risk stratification (total death and total death or development of severe HF, defined as Killip classes III-IV at admission and/or NYHA III-IV at follow-up) of ACS patients with anemia vs. without anemia as well as with lower Killip classes (≤2) vs. higher Killip classes (≥3). The results were considered statistically significant for p<0.05 and a 95% confidence interval.
ResultsSample characterizationOver the period from July 2013 to June 2015, 976 patients diagnosed with ACS were admitted to our coronary unit. Patients with chronic inflammatory disease (n=26), liver disease (n=2), end-stage CKD (n=2), malignant tumors (n=43) and/or on iron or erythropoietin supplementation (n=3) at admission were excluded. Patients for whom there was nodata on serum iron, ferritin and TIBC level (n=86) tests were also excluded. Some patients had more than one exclusion criterion; this amounted to a total of 159 excluded patients. The comparison of baseline characteristics between included and excluded patients is summarized in Table 1.
Differences in baseline characteristics between included and excluded patients.
| Patients included (n = 817) | Patients excluded (n = 159) | p | |
|---|---|---|---|
| Demographic | |||
| Age (years) | 63±13 | 66±13 | 0.012 |
| Male (%) | 78.6 | 80.5 | 0.662 |
| CVRF (%) | |||
| Diabetes | 27.5 | 34.6 | 0.089 |
| Hypertension | 61.2 | 64.8 | 0.447 |
| Dyslipidemia | 55.3 | 55.3 | 1.000 |
| Active smoker | 31.7 | 25.8 | 0.166 |
| Ex-smoker | 23.3 | 26.6 | 0.426 |
| CV history (%) | |||
| AMI | 13.2 | 17.6 | 0.181 |
| Angina | 12.7 | 17.6 | 0.117 |
| Stroke | 7.2 | 10.7 | 0.183 |
| Peripheral artery disease | 3.4 | 8.8 | 0.004 |
| Carotid disease | 1.5 | 0.0 | 0.253 |
| PCI | 9.9 | 11.9 | 0.528 |
| CABG | 4.3 | 6.3 | 0.370 |
| AF | 4.7 | 6.3 | 0.501 |
| Valve disease | 2.9 | 7.5 | 0.003 |
| DCM | 11.0 | 13.8 | 0.558 |
| CKD | 3.7 | 10.7 | <0.001 |
| Usual Medication (%) | |||
| AAS | 23.1 | 32.7 | 0.014 |
| Clopidogrel | 8.5 | 11.9 | 0.209 |
| Hypocoagulants | 3.8 | 4.4 | 0.890 |
| ACE/ARA | 32.3 | 41.5 | 0.031 |
| BB | 23.0 | 22.0 | 0.864 |
| Statin | 36.4 | 45.3 | 0.042 |
| CCB | 20.0 | 20.8 | 0.902 |
| Diuretic | 23.4 | 32.1 | 0.027 |
| Aldosterone antagonist | 1.7 | 1.9 | 0.879 |
| Ivabradine | 1.8 | 1.3 | 0.858 |
| Nitrate | 6.0 | 6.9 | 0.793 |
| Clinical Presentation (%) | |||
| STEMI | 41.5 | 37.1 | 0.346 |
| NSTEMI | 53.2 | 56.0 | 0.586 |
| Unstable Angina | 5.3 | 6.9 | 0.475 |
Legend: AF: atrial fibrillation; ACE: angiotensin-converting enzyme inhibitors; ARA: angiotensin II receptor antagonists; AF: atrial fibrillation; AMI: acute myocardial infarction; BB: beta blocker; CCB: calcium channel blocker; CABG: coronary artery bypass grafting; DCM: dilated cardiomyopathy; CVRF: cardiovascular risk factor; NSTEMI: non-ST elevation myocardial infarction; PCI: primary coronary intervention; STEMI: ST-elevation myocardial infarction.
A total of 817 patients were included, 78.6% male, with a mean age of 63 ± 13 years. The most frequent CVRF was hypertension (61.2%), followed by dyslipidemia (55.3%); 13.2% of the individuals in the sample already had a history of AMI. The most common form of presentation of ACS was non-ST elevation myocardial infarction (NSTEMI) (53.2%). It is noteworthy that the statistically significant differences founded between the two groups were mostly directly related to the exclusion criteria.
Characterization of the two sample groupsGroup 1 consisted of 519 individuals (64%) who did not have IDef and Group 2 consisted of 298 individuals (36%) who had IDef. Among the patients withIDef, 165 (55%) had absolute and 133 (45%) had functional IDef. The correlation between demographic characteristics, presence of CVRF, CV history and usual medication between the two groups is shown in Table 2.
Comparison of baseline characteristics between the two groups.
| Patients without iron deficiency (n = 519) | Iron-deficient patients (n = 298) | p | |
|---|---|---|---|
| Demographic | |||
| Age (years) | 62±13 | 65±13 | 0.002 |
| Gender Male (%) | 86.5 | 64.8 | <0.001 |
| CVRF (%) | |||
| Diabetes | 22.0 | 37.2 | <0.001 |
| Hypertension | 57.6 | 67.4 | 0.007 |
| Dyslipidemia | 54.5 | 57.1 | 0.525 |
| Active Smoker | 35.8 | 24.5 | 0.001 |
| Ex-smoker | 23.8 | 22.3 | 0.707 |
| CV history (%) | |||
| AMI | 11.4 | 16.4 | 0.051 |
| Angina | 11.6 | 14.8 | 0.225 |
| Stroke | 5.8 | 9.7 | 0.051 |
| Peripheral artery disease | 2.5 | 5.0 | 0.087 |
| Carotid disease | 2.2 | 0.3 | 0.081 |
| PCI | 8.9 | 11.7 | 0.228 |
| CABG | 2.9 | 6.7 | 0.016 |
| AF | 4.0 | 5.7 | 0.362 |
| Valve disease | 1.7 | 5.0 | 0.082 |
| DCM | 8.7 | 15.5 | 0.051 |
| CKD | 3.3 | 4.4 | 0.547 |
| Usual medication (%) | |||
| AAS | 20.2 | 28.5 | 0.009 |
| Clopidogrel | 6.9 | 11.1 | 0.053 |
| Hypocoagulants | 2.3 | 6.4 | 0.006 |
| ACE/ARA | 27.2 | 41.6 | <0.001 |
| BB | 21.0 | 26.5 | 0.086 |
| Statin | 35.3 | 38.6 | 0.381 |
| CCB | 19.1 | 21.8 | 0.396 |
| Diuretic | 19.7 | 30.2 | 0.001 |
| Aldosterone antagonist | 1.2 | 2.7 | 0.180 |
| Ivabradine | 1.3 | 2.7 | 0.272 |
| Nitrate | 4.6 | 8.7 | 0.028 |
AF: atrial fibrillation; ACE: angiotensin-converting enzyme inhibitors; ARA: angiotensin II receptor antagonists; AF: atrial fibrillation; AMI: acute myocardial infarction; BB: beta blocker; CCB: calcium channel blocker; CABG: coronary artery bypass grafting; DCM: dilated cardiomyopathy; CVRF: cardiovascular risk factor; NSTEMI: non-ST elevation myocardial infarction; PCI: primary coronary intervention; STEMI: ST-elevation myocardial infarction.
The population in group 1 had a mean age of 62±13 years, and the population in group 2 was significantly older, with a mean age of 65±13 years (t(816)=−3141, p=0.002 d=−0.230). In group 1, they were more often male (χ2(1, N=817)=51908, p<0.001, Φ=−0255).
The most common CVRF was hypertension in both groups, 57.6% in group 1 and 67.4% in group 2. A higher prevalence of hypertension and diabetes was observed in group 2 compared to group 1 (χ2(1, N=817)=7309, p=0.007, Φ=0.097 and χ2(1, N=817)=21 398, p<0.001, Φ=0.165, respectively). Group 1 showed a higher prevalence of active smokers (χ2(1, N=817)=10 729, p=0.001, Φ=−0.117). The remaining CVRFs were similar between the groups.
The CV history of patients in both groups was similar, however, patients of group 2 had undergone coronary artery bypass grafting more frequently (χ2(1, N=817)=5.842, p=0.016, Φ=0.091). Group 2 patients were more frequently medicated with acetylsalicylic acid (χ2(1, N=817)=6836, p=0.009, Φ=0.094), hypocoagulants (χ2(1, N=817)=7487, p=0.006, Φ=0.102), angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists (χ2(1, N=817)=17366, p<0.001, Φ=0.149), diuretic (χ2(1, N=817)=11 037, p=0.001, Φ=0.119) and nitrates (χ2(1, N=817)=4849, p=0.028, Φ=0.082).
Clinical presentationThe comparison of data on patient clinical presentation in both groups is summarized in Table 3.
Comparison of clinical presentation between the groups.
| Patients without iron deficiency (n = 519) | Iron-deficient patients (n = 298) | p | |
|---|---|---|---|
| ACS Type (%) | |||
| STEMI | 43.9 | 36.9 | 0.059 |
| NSTEMI | 50.6 | 58.1 | 0.044 |
| Unstable angina | 5.5 | 5.0 | 0.952 |
| Vital signs | |||
| SBP(mmHg) | 132 ± 27 | 130 ± 27 | 0.358 |
| DBP (mmHg) | 78 ± 16 | 77 ± 15 | 0.202 |
| HR (bpm) | 77 ± 18 | 77 ± 18 | 0.754 |
| Killip (%) | |||
| Class II-IV | 12.1 | 21.8 | <0.001 |
| Class III-IV | 2.7 | 6.4 | 0.017 |
| Anemia (%) | 8.5 | 20.1 | <0.001 |
| GFR <60 ml/min (%) | 17.9 | 30.2 | <0.001 |
| Pro-BNP on admission | 2077.4 | 3878.8 | <0.001 |
Legend: ACS: acute coronary syndrome; DBP: diastolic blood pressure; GFR: glomerular filtration rate; NSTEMI: Non-ST elevation myocardial infarction; Pro-BNP: pro-brain natriuretic peptide; SBP: systolic blood pressure; STEMI: ST elevation myocardial infarction.
The most common form of ACS presentation was NSTEMI, being more frequent in group 2 (χ2(1, N=817)=4.061, p=0.044, Φ=0.073)
Vital sign data at admission were similar between the groups; however, patients in group 2 presented more often with higher Killip classes (χ2(1, N=817)=12.685, p<0.001, Φ=0.128), anemia and glomerular filtration rate <60 ml/min (χ2(1, N=817)=22.116, p<0.001, Φ=0.168 and χ2(1,N=817)=15 730p<0.001, Φ=0.142).
Complementary diagnostic and therapeutic methods resultsAngiographic and echocardiographic data are shown in Table 4. There were no significant differences in whether or not cardiac catheterization was performed in the patients of both groups.
Angiographic and echocardiographic data per group.
| Patients without iron deficiency (n=519) | Iron-deficient patients (n=298) | p | |
|---|---|---|---|
| Catheterization performed (%) | 99.2 | 98.7 | 0.672 |
| Coronary heart disease (%) | |||
| No disease | 1.9 | 1.7 | 1.000 |
| 1 vessel | 57.5 | 47.4 | 0.008 |
| 2 vessels | 24.9 | 24.2 | 0.358 |
| 3 vessels | 16.5 | 26.6 | 0.001 |
| >2 vessels | 40.4 | 49.8 | 0.012 |
| Primary PCI (%) | 55.0 | 51.8 | 0.489 |
| Revascularization (%) | 87.7 | 81.5 | 0.022 |
| PCI | 81.5 | 73.7 | 0.012 |
| PCI 2nd time | 10.4 | 10.9 | 0.902 |
| CABG | 8.7 | 10.5 | 0.468 |
| Echocardiogram | |||
| LVEF (mean ± standard deviation) | 47.8±8.8 | 42.9±10.3 | <0.001 |
| Depression of LV function (LVEF <50%) (%) | 60.9 | 66.4 | 0.136 |
| Moderate-severe LV Depression (LVEF <40%) (%) | 22.3 | 34.2 | <0.001 |
| Changes in LV kinetics (%) | 87.5 | 89.6 | 0.431 |
| RV Function (%) | 3.1 | 6.4 | 0.039 |
CABG: coronary artery bypass grafting; LVEF: left ventricular ejection fraction; LV: Left ventricular; PCI: percutaneous coronary intervention; RV: right ventricular.
In group 1, one vessel disease was more frequent (χ2(1, N=817)=7105, p 0.008, Φ=−0.097) while in group 2, three vessels disease was more frequent (χ2(1, N=817)=11 284, p=0,001, Φ=0.122). It was also observed that group 1 underwent revascularization procedures more frequently (χ2(1, N=817)=5225, p=0.022, Φ=−0.084).
Patients with IDef presented more frequently with moderate and severe LV function depression (χ2(1, N=817)=12 830, p<0.001, Φ=0.130) and RV function depression (χ2(1, N=817)=4.249, p=0.039, Φ=0.079).
In-hospital progressionRegarding in-hospital therapy, some drugs were used more frequently in group 2, such as diuretics (χ2(1, N=817)=18 097, p<0.001, Φ=0.152) aldosterone antagonists (χ2(1, N=817)=18.097, p<0.001, Φ=0.152) and nitrates (χ2(1, N=817)=4.348, p=0.037, Φ=0.076). There were no statistically significant differences in the remaining drugs.
Compared to group 1, group 2 achieved higher Killip classes (χ2(1, N=817)=19255, p<0.001, Φ=0.156). There were no other significant differences between the groups in the occurrence of the remaining in-hospital events.
All data on therapy and the occurrence of in-hospital events are presented in Table 5.
Comparison of in-hospital evolution between groups.
| Patients without iron deficiency (n = 519) | Iron-deficient patients (n = 298) | p | |
|---|---|---|---|
| In-hospital therapy | |||
| UFH | 38.7 | 33.9 | 0.193 |
| LMWH | 60.3 | 66.1 | 0.116 |
| IIb/IIIa inhibitors | 8.1 | 6.4 | 0.447 |
| AAS | 99.2 | 99.3 | 1.000 |
| Clopidogrel | 98.3 | 99.7 | 0.156 |
| BB | 89.8 | 89.6 | 1.000 |
| ACE/ARA | 92.7 | 93.6 | 0.713 |
| Statin | 98.3 | 99.0 | 0.596 |
| Diuretics | 20.4 | 34.2 | <0.001 |
| Aldosterone antagonists | 17.1 | 30.2 | <0.001 |
| CCB | 9.2 | 10.4 | 0.679 |
| Nitrates | 14.3 | 20.1 | 0.037 |
| Inotropics | 3.7 | 4.4 | 0.756 |
| In-hospital events | |||
| Killip Class (%) | |||
| Class II-IV | 19.3 | 33.2 | <0.001 |
| Class III-IV | 5.4 | 9.4 | 0.042 |
| HF (%) | 18.1 | 32.2 | <0.001 |
| Angina (%) | 2.5 | 3.7 | 0.452 |
| Re-infarction (%) | 1.5 | 0.0 | 0.074 |
| Transfusion (%) | 1.0 | 0.7 | 0.967 |
| Death (%) | 1.3 | 1.0 | 0.922 |
| Malignant arrhythmias (%) | 5.2 | 4.4 | 0.673 |
| 2nd and 3rd degree AVB (%) | 2.9 | 4.4 | 0.453 |
| Respiratory infection (%) | 4.4 | 5.4 | 0.642 |
ACE: angiotensin-converting enzyme inhibitors; AVB: atrioventricular block; BB: beta blocker; CCB: calcium channel blocker; HF: heart failure; LMWH: low molecular weight heparin; UFH: unfractionated heparin.
Information on the follow-up of patients in both groups is summarized in Table 6. Average follow-up was 738.77 days.
Comparison of events during follow-up between groups.
| Patients without iron deficiency (n=519) | Iron-deficient patients (n=298) | p | |
|---|---|---|---|
| Death (%) | 6.3 | 12.6 | 0.004 |
| CV Cause | 3.2 | 4.5 | 0.424 |
| Non-CV Cause | 1.8 | 4.5 | 0.040 |
| Unknown cause | 1.4 | 3.5 | 0.086 |
| Stroke (%) | 0.8 | 0.7 | 1.000 |
| Re-infarction (%) | 4.5 | 3.8 | 0.777 |
| Angina (CCS > I) (%) | 4.6 | 7.0 | 0.197 |
| HF (NYHA III-IV) (%) | 5.3 | 10.5 | 0.011 |
| Total death/serious HF(%) | 13.8 | 25.3 | <0.001 |
| Hospital Readmissions | 9.8 | 13.7 | 0.048 |
Legend: CCS: Canadian Vascular Society; CV: cardiovascular.
Group 2 patients had a higher rate of events at follow-up, especially higher mortality (χ2(1, N=792)=8352, p=0.004, Φ=0.107) and NYHA III-IV HF (χ2(1, N=792)=6515, p=0.011, Φ=0.096). The hospital readmission rate was also higher in the group with IDef (9.8% vs. 13.7%, p=0.048). The occurrence of reinfarction, angina and stroke was similar between the groups.
Assessment of prognosisIndependent predictors of prognosis are shown in Table 7. The analysis of factors associated with death or development of severe HF is in Appendix V.
Independent predictors of death/severe heart failure.
| B | IF | X2 Wald | HR | 95% CI | p | |
|---|---|---|---|---|---|---|
| Age >60 years | 0.233 | 0.240 | 0.942 | 1.262 | 0.79–2.02 | 0.332 |
| GFR <60 ml/min | 0.854 | 0.244 | 12.264 | 2.349 | 1.46–3.79 | <0.001 |
| Revascularization | −0.558 | 0.266 | 4.402 | 0.572 | 0.34–0.96 | 0.036 |
| Iron deficiency | 0.510 | 0.207 | 6.090 | 1.666 | 1.11–2.50 | 0.014 |
| Anemia | 0.729 | 0.261 | 7.815 | 2.072 | 1.24–3.45 | 0.005 |
| LV dysfunction | 1.477 | 0.275 | 28 850 | 4.381 | 2.56–7.51 | <0.001 |
Legend: GFR: glomerular filtration rate; HR: hazard ratio; LV: left ventricular; CI: confidence interval CV: cardiovascular.
Iron deficiency was an independent predictor of death or development of severe HF. The remaining independent predictors of these events were renal dysfunction (GFR<60 ml/min), absence of revascularization, anemia and LV dysfunction.
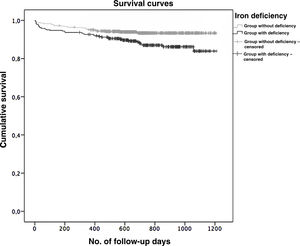
Survival analysisIn this study, 97% of the patients in the sample were followed up for at least one year. Kaplan–Meyer survival curves were obtained for the two groups under study, which can be seen in Graph 1. There was a significant difference between the two groups in terms of survival after the ACS event, which was greater superior in those who did not present with IDef (Log-Rank Test, p=0.003).
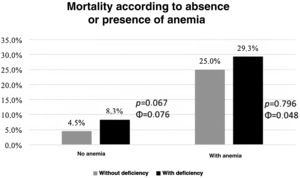
Risk stratificationAs can be seen in Graph 2, patients with IDef had higher mortality rate regardless of the presence or absence of anemia, although this difference was not statistically significant.
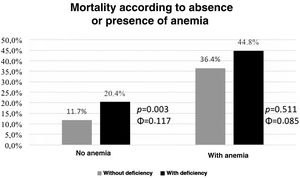
As illustrated in Graph 3, the presence of IDef enabled the additional stratification of patients without anemia at admission (χ2(1, N=693)=8805, p=0.003, Φ=0.117) in terms of the occurrence of death or severe HF. In patients with anemia on admission, IDef did not enable additional stratification.
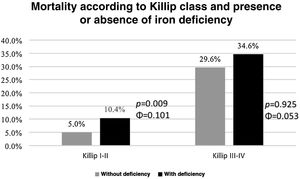
Similarly, the presence of IDef allowed additional stratification of patients with lower Killip classes (≤2); in these patients, there was higher mortality in those with IDef (χ2(1, N=739)=6762, p=0.009, Φ=0.101) (Graph 4). In patients with higher Killip classes (≥3), patients with IDef had higher mortality rate, however this difference did not achieve statistical significance.
DiscussionThe main finding of this study was that IDef was an independent predictor of death or severe HF in a population of ACS patients.
In addition,IDef enabled the additional stratification of the prognosis of the patients without anemia for the occurrence of death or severe HF and of those with lower Killip classes (≤2) in terms of occurrence of death.
Additionally, this study showed that IDef is a prevalent condition in a population of ACS patients.
The results of this study corroborate what is currently known in the HF patient population, in which IDef, independent of anemia, is associated with higher morbidity and mortality, a higher number of hospital admissions, and worse quality of life.2,4,5,18
For this reason, current HF guidelines recommend laboratory testing for all patients with HF to diagnose IDef and to start iron supplementation in those with reduced EF, preferably intravenously and with carboxymaltose, regardless of the presence of anemia.3,21,23
Data in the literature are still scarce regarding the IDef in the population of patients with ACS. One of the first studies published on this topic showed that IDef was a determinant of functional capacity and health-related quality of life 30 days after ACS.17 This study included a small number of patients (n=244) and involved a short 30-day period follow-up. It focused on the assessment of functional capacity and quality of life, with and IDef was a predictor of functional capacity and quality of life but not of cardiovascular morbidity and mortality.17 The present study, on the other hand, included a larger sample, a short and long term prognostic assessment (minimum follow-up of one year) and demonstrated the impact of IDef on the occurrence of death, and was and independent predictor of death and severe HF together with other well-known predictors in ACS.25,26,32–34 In that study, the prevalence of IDfe was 46%,17 slightly higher than that reported in our study (36%).
One of the reasons for the high prevalence of IDef in patients with ACS, up to 61% in a recent study, is related to blood loss during invasive procedures and the use of anti-thrombotic therapy.16,20,29 It has been shown that drops in hemoglobin and in the serum iron levels increase mortality. However, in that study it was not possible to show IDef as an independent predictor of death.16
The prevalence of anemia in this study was 4.5%, lower than that found in our study (12.7%). It is known that anemia is a very common condition in hospitalized patients; it cited as a factor of poor prognosis and an important predictor of death both in patients with ACS and in those with HF.16,21,33,35,36,37–40 Its prevalence at admission in patients with ACS seems to range from 17%–28%, increasing over the course of hospitalization.16
In the last two years, some studies assessing the role of IDef in the prognosis of patients hospitalized with ACS have been published.39–42One such study published in 2018 included 836 patients, 29.1% of whom had IDef, with a higher prevalence in women and in patients with anemia.41 After a mean follow-up of four years, IDef was shown to be a strong predictor of non-fatal AMI and cardiovascular mortality in this patient population. In view of results from this study and the evidence already available in the literature on the efficacy of iron supplementation in HF with reduced EF, the same authors conducted a multicenter randomized controlled study, the CAYAN trial (Comprehensive management of iron deficiency in myocardial infarction).43 In this trial, patients with clinically relevant AMI and concomitant IDef were randomized to receive iron carboxymaltose or placebo (saline solution). All patients underwent cardiac magnetic resonance imaging at baseline and follow-up to assess the primary endpoint (change in LVEF at baseline and fourth month of follow-up). It should be noted that the aforementioned published studies assessed the IDef at baseline as a landmark to define the groups with and without IDef and to assess the respective prognoses. As in this study, no analysis of iron supplementation at follow-up and its possible correction, as well as its influence on long term prognosis was performed, so that the results of this trial may be important in changing the approach of patients with ACS and IDef.
In another study published in 2019, which included 420 patients with STEMI, the association of IDef with in-hospital mortality and Killip class 3 and 4 was assessed. In this study, a paradoxical result was obtained, in which patients with IDef had better in-hospital prognosis.42
In the present study, anemia was also a predictorof poor prognosis. In order to assess whether the relationship between IDef and prognosis was independent from anemia, in addition to the logistic regression analysis previously described, we divided the sample into two groups (with anemia and without anemia at admission) and compared the incidence of death in patients with and without IDef. It was found that, regardless of the presence of anemia, patients with IDef had higher mortality than patients without IDef. The differences were however not statistically significant, which is possibly related to the low number of deaths in the sample. However, in the stratification of risk of death or development of severe HF, the group without anemia but with IDef had a higher rate of events than those without IDef. A possible explanation for this result may be that iron is not only important in hematopoiesis, but also affects cellular metabolism due to its extensive action in various enzymatic reactions, induction of oxidative stress, and may even cause structural changes in cardiomyocytes, affecting ventricular remodeling and worsening LV function, which consequently leads to worse prognosis.4,8,11,14,15 This result corroborates the HF guidelines, which recommend iron suplementation in patients with IDef even in the absence of anemia. In patients with anemia, there was a higher rate of death or severe HF in patients with IDef, although it was not statistically significant.
Similarly, in order to study the role of IDef in patients with ACS according to the presence or absence of HF, the sample was divided into two groups: patients with Killip Class I–II and those with Killip Class III–IV. In those with Killip Class I–II, IDef enabled additional stratification according to risk of death, revealing a higher mortality rate in those with IDeF. One of the possible explanations for this outcome may be related to alterations in the immune cell response triggered by IDef, in addition to the deranged energy metabolisms already described.1,3,4,8,11 This result highlights the importance of IDef even in the absence of HF or mild HF in the ACS patient population. In patients with Killip Class III–IV, although there was a higher mortality in those with IDef, this result was not statistically significant, which may be due to the low number of patients included in this group and/or the low number of events that occurred.
Different from the HF guidelines, the ACS guidelines do not recommend the evaluation of the iron kinetics to search for IDef.25,26 This study shows the importance of descriminate these patients and futute studies may show the impact of iron suplementation in their prognosis.
LimitationsThis is a single-center retrospective study with all the known inherent limitations. In addition, only one set of iron kinetic parameter tests was used, making it impossible to assess their evolution over time. Also, no therapeutic follow-up assessment was carried out, especially regarding possible iron supplementation. Finally, some patients were excluded due to the absence of iron kinetic parameters, which may be considered as study bias.
ConclusionThis study showed that IDef is a prevalent condition in a population of ACS patients and that, in this population, it was an independent predictor of death or severe HF. Moreover, IDef allowed additional stratification of patients without anemia on admission based on the occurrence of death or severe HF and of those with lower Killip classes (≤2) based on the occurrence of death.
These results may have an impact on clinical practice, especially if further studies attest to an improved prognosis for these patients after iron supplementation.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Silva C, Martins J, Campos I. Arantes C, Braga CG, Salomé N, et al., Impacto prognóstico do défice de ferro nas síndromes coronárias agudas, Rev Port Cardiol. 2021;40:525–535.