Acromegaly is a rare, chronic and slowly developing endocrine disorder caused by hypersecretion of human growth hormone and consequently of insulin-like growth factor-1 during adulthood. The present study was conducted to assess mitral annular (MA) size and function between acromegalic patients and age- and gender-matched healthy controls by three-dimensional speckle-tracking echocardiography (3D-STE). It also aimed to examine whether activity of the disease has any effect on MA parameters.
MethodsThis study included 27 patients with acromegaly, three of whom were excluded due to inferior image quality. The mean age of the remaining 24 patients was 55.7±14.0 years and seven were male. Complete two-dimensional Doppler echocardiography and 3D-STE were performed in all cases.
ResultsSignificantly increased end-diastolic and end-systolic MA diameter (2.81±0.36 cm vs. 2.44±0.34 cm and 2.00±0.32 cm vs. 1.65±0.37 cm, respectively), area (9.67±2.33 cm2 vs. 7.38±1.93 cm2 and 5.14±1.62 cm2 vs. 3.74±1.19 cm2, respectively) and perimeter (11.76±1.42 cm vs. 10.27±1.33 cm and 8.61±1.23 cm vs. 7.36±1.10 cm, respectively) were demonstrated in acromegalic patients compared with control subjects. MA functional parameters were not significantly altered compared to those of healthy individuals.
ConclusionsMA dilation could be seen in acromegaly regardless of its activity. Acromegaly is not associated with MA functional impairment.
A acromegalia é uma doença endócrina rara, crónica e de desenvolvimento lento, causada pela hipersecreção da hormona do crescimento humano e, consequentemente, do fator de crescimento semelhante à insulina-1 durante a idade adulta. O presente estudo foi conduzido para avaliar o tamanho e a função do anel mitral (MA) nos doentes com acromegalia e controles saudáveis emparelhados por idade e sexo, por meio de ecocardiografia tridimensional com speckle-tracking (3DSTE). O objetivo foi também examinar se a atividade da doença tem algum efeito nos parâmetros da MA.
MétodosEste estudo incluiu 27 pacientes com acromegalia, três dos quais tiveram de ser excluídos devido à qualidade inferior de imagem. A média de idade dos 24 pacientes restantes foi de 55,7 ± 14,0 anos (7 homens). Foram realizados em todos os casos uma ecocardiografia Doppler bidimensional completa e um 3DSTE.
ResultadosQuando comparados com os controlos, os doentes acromegálicos apresentaram diâmetros diastólico e sistólico finais significativamente dilatados (2,81 ± 0,36cm versus 2,44 ± 0,34cm e 2,00 ± 0,32cm versus 1,65 ± 0,37cm, respetivamente), assim como a área valvular (9,67 ± 2,33cm2 versus 7,38 ± 1,93cm2 e 5,14 ± 1,62cm2 versus 3,74 ± 1,19cm2, respetivamente) e o perímetro (11,76 ± 1,42cm versus 10,27 ± 1,33cm e 8,61 ± 1,23cm versus 7,36 ± 1,10cm, respetivamente) Os parâmetros funcionais da MA não estavam significativamente alterados em comparação com indivíduos saudáveis.
ConclusõesA dilatação do MA pode ser observada na acromegalia, independentemente de sua atividade. A acromegalia não está associada ao comprometimento funcional da MA.
Acromegaly is a rare, chronic and slowly developing endocrine disorder caused by hypersecretion of human growth hormone (hGH) and consequently of insulin-like growth factor-1 (IGF-1) during adulthood.1 Acromegalic cardiomyopathy is reported to be one of the most important complications of acromegaly and cardiovascular events may be linked to these hormones.2–4 The saddle-shaped mitral annulus (MA) plays an important role in the filling and emptying of the left heart chambers. Perturbations of MA geometry and function can be seen in a number of diseases, particularly in valvular disorders and atrial fibrillation.5 However, some non-valvular disorders have also been found to be associated with MA morphological and functional alterations.6–8 The present study was conducted to compare MA size and functional properties between acromegalic patients and age- and gender-matched healthy controls by three-dimensional speckle-tracking echocardiography (3D-STE).
MethodsPatient populationThe study included 27 patients with acromegaly, three of whom were excluded due to inferior image quality. The mean age of the remaining 24 patients was 55.7±14.0 years and seven were male. Their results were compared to 38 healthy controls (mean age: 53.4±4.4 years, 16 male). For all patients and controls, 3D-STE was performed in addition to routine two-dimensional Doppler echocardiography. The diagnosis of acromegaly was based on and its activity was defined in accordance with current guidelines, as detailed previously.1 All acromegalic patients were included in the Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography in Pathological cases (MAGYAR-Path Study). All patients gave their informed consent. The study was approved by the human research committee at the University of Szeged and followed the ethical guidelines of the 1975 Declaration of Helsinki.
Two-dimensional Doppler echocardiographyA Toshiba Artida™ echocardiograph (Toshiba Medical Systems, Tokyo, Japan) with a PST-30SBP phased-array transducer (1-5 MHz) was used to perform standard two-dimensional (2D) Doppler transthoracic echocardiographic examinations following recent guidelines.9 A qualitative (0-4) scale was used to grade mitral regurgitation, where 0 represents no regurgitation and 4 stands for the most severe regurgitation possible.
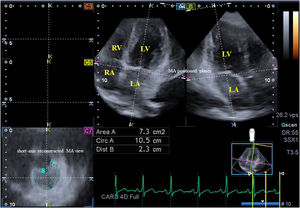
Three-dimensional speckle-tracking echocardiography3D-STE was performed with the same Toshiba Artida™ echocardiograph (Toshiba Medical Systems, Tokyo, Japan) attached to a PST-25SX matrix-array transducer.10 An apical window was used for 3D-STE data acquisition, in which six wedge-shaped subvolumes were acquired during a single breath-hold in sinus rhythm, from which a full-volume 3D dataset was created automatically. Offline analysis of the 3D datasets was carried out using 3D Wall Motion Tracking software, version 2.7 (Toshiba Medical Systems, Tokyo, Japan). Apical 2-chamber and 4-chamber views and three short-axis views – basal (C7), midventricular and apical left ventricular (LV) levels – were selected at end-diastole by the software. Following optimization of imaging planes in apical 4- and 2-chamber views on the edges of the MA, several MA morphological parameters were measured in the C7 short-axis view. The following morphological parameters were measured at end-diastole (just before mitral valve closure) and end-systole (just before mitral valve opening) (Figure 1)6–8:
- -
MA diameter (MAD), defined as the perpendicular line drawn from the peak of MA curvature to the middle of the straight MA border (anteroposterior diameter);
- -
MA area (MAA) measured by planimetry;
- -
MA perimeter (MAP) measured by planimetry.
Images from the three-dimensional full-volume dataset showing the mitral annulus in a patient with acromegaly including apical 4-chamber (A) and 4-chamber (B) long-axis views and a cross-sectional view at the level of the mitral annulus (C7) optimized in long-axis views. Area: mitral annular area; Circ: mitral annular perimeter; Dist: mitral annular diameter; LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.
Using the above parameters, the following MA functional properties were calculated:
- -
MA fractional shortening (MAFS), defined as ([end-diastolic MAD−end-systolic MAD]/end-diastolic MAD)×100,
- -
MA fractional area change (MAFAC), defined as ([end-diastolic MAA−end-systolic MAA]/end-diastolic MAA)×100.
All data were presented as number (percentage) or mean ± standard deviation. Differences in values were considered to be statistically significant with p<0.05. Pearson's coefficient was used for correlation between variables. Bland and Altman's method was used for studying intra- and interobserver agreement. Data analyses were performed using RStudio Team statistical software (RStudio: Integrated Development for R. RStudio, Inc., Boston, MA, 2015).
ResultsClinical, laboratory, therapeutic and two-dimensional echocardiographic dataSignificant differences were demonstrated in most echocardiographic parameters between all acromegalic patients and healthy control subjects. Differences between active and inactive acromegalic patients and controls are presented in Table 1. IGF-1 levels and indices and early transmitral flow velocity differed significantly between active and inactive acromegalic subjects (Table 1).
Clinical, demographic and two-dimensional echocardiographic data in acromegalic patients and matched controls.
| Controls (n=38) | All acromegalic patients (n=24) | Active acromegalic patients (n=12) | Inactive acromegalic patients (n=12) | |
|---|---|---|---|---|
| Clinical and demographic data | ||||
| Age, years | 53.4±4.4 | 55.7±14.0 | 59.8±10.1 | 51.5±16.5 |
| Male gender, % | 16 (42) | 7 (29) | 4 (33) | 3 (29) |
| Hypertension, % | 0 (0) | 12 (50) | 7 (58) | 5 (42) |
| Diabetes, % | 0 (0) | 3 (13) | 3 (25) | 0 (0) |
| Hypercholesterolemia, % | 0 (0) | 9 (38) | 5 (42) | 4 (33) |
| Laboratory findings | ||||
| Serum hHG, ng/ml | – | 5.0±6.2 | 5.4±3.8 | 4.4±8.5 |
| Serum IGF-1, ng/ml | – | 313±200 | 425±199 | 179±92b |
| Serum IGF-1 index | – | 1.34±0.90 | 1.83±0.90 | 0.74±0.40b |
| Therapy | ||||
| Somatostatin analogue, % | 0 (0) | 8 (33) | 4 (33) | 4 (33) |
| Bromocriptine, % | 0 (0) | 8 (33) | 5 (42) | 3 (25) |
| Pegvisomant, % | 0 (0) | 1 (4) | 1 (8) | 0 (0) |
| Hypophysectomy, % | 0 (0) | 9 (38) | 5 (42) | 4 (33) |
| Two-dimensional echocardiography | ||||
| LA diameter, mm | 39.0±4.0 | 42.1±5.6a | 41.2±6.4 | 43.3±4.5a |
| LVEDD, mm | 48.3±4.1 | 51.1±5.4a | 49.9±5.2 | 52.8±5.6a |
| LVEDV, ml | 111.3±36.3 | 128.3±28.6a | 122.6±24.8 | 136.1±33.1a |
| LVESD, mm | 32.4±4.6 | 32.0±5.0 | 30.6±4.9 | 33.9±4.7 |
| LVESV, ml | 39.4±13.7 | 42.7±15.7 | 38.5±14.6 | 48.4±16.3 |
| IVS, mm | 9.2±1.3 | 10.1±1.5a | 10.5±1.8a | 9.6±0.9 |
| LV PW, mm | 9.5±1.3 | 10.9±1.9a | 10.9±2.1a | 10.9 ±1.8a |
| LVEF, % | 64.4±3.4 | 66.7±7.7 | 68.1±8.7 | 64.7±6.0 |
| E, cm/s | 73.0 ±16.7 | 62.3±10.4a | 57.8±8.2a | 68.6±10.2b |
| A, cm/s | 68.6±17.3 | 79.3±15.7a | 77.7±14.9a | 81.4±17.6a |
| E/A | 1.10±0.25 | 0.81±0.20a | 0.76±0.17a | 0.88±0.23a |
| Mean MR grade | 0.2±0.3 | 0.7±0.5 | 0.5±0.5 | 0.8±0.6 |
| 0, % | 24 (0) | 18 (75) | 10 (83) | 8 (67) |
| 1, % | 0 (0) | 5 (21)a | 2 (17) | 3 (25)a |
| 2, % | 0 (0) | 1 (4) | 0 (0) | 1 (8) |
E/A: ratio of early and late diastolic transmitral flow velocity; hGH: human growth hormone; IGF-1: insulin-like growth factor-1; IVS: interventricular septum; LA: left atrial; LV: left ventricular; LVEDD: end-diastolic diameter; LVEDV: end-diastolic volume; LVEF: left ventricular ejection fraction; LVESD: end-systolic diameter; LVESV: end-systolic volume; MR: mitral regurgitation; PW: posterior wall.
Significantly increased MA dimensions were demonstrated in acromegalic patients. MA functional properties did not differ between patients with acromegaly and age- and gender-matched controls. No differences in MA data were seen according to the activity of acromegaly (Table 2).
Comparison of three-dimensional speckle-tracking echocardiography-derived mitral annular morphological and functional parameters between patients with acromegaly and controls.
| Controls (n=38) | All acromegalic patients (n=24) | Active acromegalic patients (n=12) | Inactive acromegalic patients (n=12) | |
|---|---|---|---|---|
| MA morphological parameters | ||||
| MAD-D, cm | 2.44±0.34 | 2.81±0.36a | 2.86±0.41a | 2.76±0.32a |
| MAA-D, cm2 | 7.38±1.93 | 9.67±2.33a | 10.12±2.58a | 9.22±2.05a |
| MAP-D, cm | 10.27±1.33 | 11.76±1.42a | 11.96±1.59a | 11.56±1.27a |
| MAD-S, cm | 1.65±0.37 | 2.00±0.32a | 2.08±0.31a | 1.91±0.31a |
| MAA-S, cm2 | 3.74±1.19 | 5.14±1.62a | 5.43±1.45a | 4.85±1.79a |
| MAP-S, cm | 7.36±1.10 | 8.61±1.23a | 8.87±1.10a | 8.35±1.34a |
| MA functional parameters | ||||
| MAFAC, % | 48.3±16.1 | 46.4±11.3 | 45.3±11.3 | 47.6±11.7 |
| MAFS, % | 32.7±13.9 | 28.6±9.4 | 26.3±11.3 | 30.9±6.7 |
MA: mitral annular; MAA-D: end-diastolic mitral annular area; MAA-S: end-systolic mitral annular area; MAD-D: end-diastolic mitral annular diameter; MAD-S: end-systolic mitral annular diameter; MAP-D: end-diastolic mitral annular perimeter; MAP-S: end-systolic mitral annular perimeter; MAFAC: mitral annular fractional area change; MAFS: mitral annular fractional shortening.
No significant correlations were detected between MA parameters and different hormone levels.
Reproducibility measurementsThe differences (mean ± standard deviation) measured by two observers for end-diastolic MAD, MAA and MAP were 0.01±0.18 cm, -0.04±0.79 cm2 and –0.19±0.98 cm, respectively, with correlation coefficients between these independent measurements of 0.97 (p<0.0001), 0.96 (p<0.0001) and 0.97 (p<0.0001), respectively (interobserver variability) at rest. The corresponding values for intraobserver variability including values obtained by two measurements by observer 1 were -0.03±0.19 cm, 0.03±1.04 cm2 and -0.01±0.95 cm, respectively, with correlation coefficients between these independent measurements of 0.98 (p<0.0001), 0.96 (p<0.0001) and 0.96 (p<0.0001), respectively.
The corresponding values for end-systolic MAD, MAA and MAP were 0.03±0.21 cm, -0.03±0.39 cm2 and 0.03±0.43 cm, with correlation coefficients of 0.97 (p<0.0001), 0.98 (p<0.0001) and 0.98 (p<0.0001), respectively (interobserver variability). The corresponding parameters for intraobserver variability including two measurements by observer 1 were -0.02±0.21 cm, -0.01±0.34 cm2 and 0.03±0.53 cm, respectively, with correlation coefficients between these independent measurements of 0.98 (p<0.0001), 0.98 (p<0.0001) and 0.97 (p<0.0001), respectively.
DiscussionIn the early stages of acromegaly, excess hGH and IGF-1 induce a hyperkinetic syndrome. Subsequently concentric hypertrophy develops, together with LV diastolic dysfunction and eventually impaired systolic function, ending in heart failure unless the excess hGH/IGF-1 is treated.3 Hypertension, arrhythmias, atherosclerosis, coronary artery disease and heart valve disease are also frequent findings in acromegaly.3
Acromegaly has been found to be associated with an increased prevalence of regurgitant valvular heart disease, which is dependent on the duration of exposure to increased hGH concentrations.11 Aortic valve regurgitation (≥trace severity) was present in 30% of acromegalic patients, and mitral regurgitation (≥moderate severity) was present in 5% of individuals with acromegaly.11 The overall prevalence of valve abnormalities is higher in both active and cured acromegalic patients. The persistence of valve disease in patients with cured acromegaly is likely to correlate with the persistence of LV hypertrophy.12
Under normal, healthy conditions, the contractile function of the myocardium in the basal regions of the left atrium and ventricle adjacent to the mitral annulus results in a sphincter-like motion of the annulus, which is an innervated fibrous ring. This sphincter-like narrowing follows the cardiac cycle.13 The circumferential fibers in the basal segment of the left atrium are positioned so that their contraction creates a centripetal force on the inner segment of the adjacent fibrous MA ring, leading to an inward motion during late diastole. The superficial oblique fibers of the LV inlet generate a torsional force on the outer segment of the mitral annulus, causing it to move inwards in systole.13 Myocardial contraction of the left atrium and ventricle following the cardiac cycle and occurring at the appropriate time are required for proper contraction of the mitral annulus.13,14
In the present study, MA dilation with preserved function was demonstrated in acromegalic patients with no significant mitral regurgitation and with no relationship to the disease's activity. In 3D-STE studies for deeper insights into acromegaly-associated abnormalities, impaired LV rotational mechanics has been found, with reduced apical LV rotation and LV twist and 20% incidence of absence of LV twist (LV rigid body rotation) in acromegalic patients.15 Moreover, increased LV radial strains have been demonstrated, together with changes in LA functional parameters, suggesting compensatory increases in LV and LA contractility to maintain LV pumping function.16–18 These results could explain both the MA dilation and its normal function, helping to elucidate these particular features of acromegalic cardiomyopathy.
These results highlight the need for reliable non-invasive assessment of MA dimensions in clinical practice. Although true MA diameter is underestimated by routine 2D echocardiography,6 transthoracic real-time 3D echocardiography (RT3DE) had been validated by magnetic resonance imaging for the assessment of MA dimensions and offers superior accuracy to 2D echocardiography, but its main limitation is that it measures the 2D-projected annulus, not its real 3D-shape.6–8 Transesophageal RT3DE (RT-3DE) using special software is able to create a virtual 3D model of the mitral valve and its annulus, but due to its semi-invasive nature its usefulness is limited.19 Although 3D-STE is optimal for simultaneous assessment of strain, rotational and volumetric parameters of the heart chambers using the same acquired 3D echocardiographic dataset, transthoracic 3D-STE has a similar ability to transesophageal RT-3DE to visualize the annulus. It is easy to use, the learning curve is short, there is no radiation, its cost is relatively low, and it can be used in asymptomatic non-cardiac patients such as individuals with acromegaly.20
Study limitationsIn our patients, acromegalic cardiomyopathy was present in both early and later stages, which could affect the results. 3D-STE is a new methodology with limited spatial (due to the limited number of piezoelectric crystals) and temporal resolution (mean 25±5 frames per second), which could affect image resolution and quality. With the presented 3D-STE-derived MA analysis, the 3D saddle shape of the annulus cannot be analyzed, only its 2D projection, which could theoretically affect the measurements. The present study did not aim to assess and compare 3D-STE-derived LV, left or right atrial volumetric and strain parameters between acromegalic patients and matched controls.
ConclusionsMA dilation was seen in acromegaly regardless of its activity. Acromegaly is not associated with MA functional impairment.
Conflicts of interestThe authors have no conflicts of interest to declare.