Direct oral anticoagulants (DOACs) changed the landscape of atrial fibrillation (AF) treatment, but also brought with them new challenges in terms of accessibility and compliance. The purpose of this study was to assess adherence to DOACs, and its determinants in a population of AF patients.
MethodsSingle-center retrospective study including all patients with non-valvular AF treated with a DOAC from the outpatient general cardiology list at a tertiary center, whose first DOAC prescription was between 1 April 2016 and August 2018. The number of pharmacy refills from the day of first prescription to 31 August 2018 was counted (by means of an electronic prescription platform). Medication refill adherence (MRA) was calculated by dividing the total days' supply by the number of days under therapy. Non-compliance was defined as MRA <90%.
ResultsA total of 264 patients (120 men, mean age 74 ± 12 years) met the inclusion criteria. The median CHA2DS2VASC score was 3 (interquartile range (IQR) 2-5) and the median HAS-BLED was 1 (IQR 1-2). Rivaroxaban, apixaban, dabigatran and edoxaban were prescribed in 45%, 41%, 24% and 13% of patients, respectively. During the study 51 patients (19%) used at least two DOACs .Patients took DOACs for a median period of 439 days (IQR 269-638), during which the included population adhered to therapy 90% of the time (IQR 75-100%). Half of the patients (51%) were classified as non-compliant; therapy duration (adjusted odds ratio 1.06 per month, 95% confidence interval (CI) 1.03-1.08, p<0.001), DOACs twice daily (adjusted OR 1.73, 95%CI 1.08-2.75, p=0.022), and higher out-of-pocket costs (adjusted OR 2.13, 95%CI 1.28-3.45, p=0.003) were independent predictors of non-compliance.
ConclusionHalf of the patients (51%) were classified as non-compliant (medication refill adherence <90%). Therapy duration, DOACs twice daily and higher out out-of-pocket costs were independent predictors of non-compliance, which could be targets to improve patient adherence.
Os anticoagulantes diretos (direct anticoagulants, DOACs) mudaram o panorama do tratamento da fibrilhação auricular (FA), trazendo novos desafios de acessibilidade e adesão terapêutica. O objetivo deste estudo foi avaliar a adesão terapêutica com DOACs, e os seus fatores determinantes, numa população de doentes com FA.
MétodosEstudo retrospetivo de centro único incluindo doentes com FA não-valvular sob terapêutica com DOAC seguidos em consulta de Cardiologia, cuja primeira prescrição de DOAC foi realizada entre 1 de Abril de 2016 e Agosto de 2018. Foram contabilizadas as embalagens de DOAC levantadas desde a primeira prescrição até 31 de Agosto de 2018, utilizando a Prescrição Eletrónica Médica. Foi calculada a adesão à terapêutica através da divisão entre o número de dias cobertos pela dispensa efetiva na farmácia e os dias sob terapêutica. Definiu-se «não adesão» como uma adesão inferior a 90%.
ResultadosForam incluídos 264 doentes (120 homens, idade média 74 ± 12 anos). O score CHA2DS2VASC mediano foi 3 (IIQ 2-5) e o score HAS-BLED mediano foi 1 (IIQ 1-2). Os DOAC rivaroxabano, apixabano, dabigatrano e edoxabano foram prescritos em 45%, 41%, 24% e 13% dos doentes, respetivamente. Ao longo do período avaliado, 51 doentes (19%) tomaram pelo menos dois DOACs diferentes. Os doentes estiveram sob DOAC durante uma mediana de 439 dias (IIQ 269-638), durante os quais aderiram à terapêutica em mediana 90% (IIQ 75-100%) do tempo. Cerca de metade (51%) dos doentes foram considerados não aderentes; a maior duração da terapêutica (OR ajustado de 1,06/mês, IC95% 1,03-1,08, p<0,001), a toma de DOAC com posologia bidiária (OR ajustado de 1,73, IC95% 1,08-2,75, p=0,022) e o pagamento de medicamentos em regime geral (OR ajustado 2,13, IC95% 1,28-3,45, p=0,003) foram preditores independentes de «não adesão».
ConclusãoMetade dos doentes (51%) com FA sob DOAC foram classificados como não aderentes (adesão <90%). A maior duração da terapêutica, a posologia bidiária e o pagamento em regime geral foram preditores independentes de não adesão, podendo constituir alvos de intervenção para melhorar o perfil de cumprimento terapêutico.
Oral anticoagulants are effective at preventing stroke in the context of non-valvular atrial fibrillation (AF). Currently, the European Society of Cardiology recommends direct oral anticoagulants (DOAC) as first-line therapy in this context,1 not only for their demonstrated efficacy and safety, but also due to the pharmacological characteristics of the class.3 However, higher costs and less contact with health care services than when vitamin K antagonists (VKA) are prescribed have been cited as potential barriers to medication adherence.
Poor adherence to chronic disease therapy is currently considered a public health problem responsible for high morbidity and mortality and extremely high financial costs.4 In the specific case of DOACs, and given their short half-life, treatment noncompliance may expose patients to an increased risk of thromboembolic events. Data published in Portugal on therapeutic adherence to anticoagulants in the context of AF are scarce, despite their relevance to clinical practice. Similarly, the impact of demographic, economic, and clinical factors on compliance is unknown. The aim of this study was to assess adherence to DOACs and the predetermining factors in a population of patients with non-valvular AF followed at the cardiology department of a tertiary hospital.
MethodsPopulationThis was a single-center retrospective study that assessed all patients with non-valvular AF under treatment with DOAC, followed at the cardiology consultations, involving a total of 15 attending cardiologists. We included patients who received their first DOAC prescription between 1 April 2016 (the start date of mandatory use of the Electronic Prescription Service (EPS) in the Portuguese National Health System)5 and August 2018, and who maintained an indication for DOAC until 31 August 2018. Patients whose DOAC therapy was started outside of this period, was discontinued (due to lack of indication for anticoagulation) before 31 August 2018 or was replaced by a VKA during the period under study (due to mandatory anticoagulation with these drugs) were excluded. Those under DOAC for an indication other than AF, and patients who had not received electronic prescriptions for at least six months were also excluded.
The demographic and clinical characteristics of each patient were identified from their medical records and the CHA2DS2VASC and HAS-BLED scores were calculated on the date of the first prescription of a DOAC. The existence of a previous prescription for VKA was also identified. Finally, each patient's drug payment category was recorded (general system or special system). Data collection and processing were approved by the local Ethics Committee.
Assessment of therapeutic adherenceTherapeutic adherence was assessed from the date of the first DOAC prescription until 31 August 2018, the date defined as the end of follow-up. The number of packs purchased and recorded in the EPS during the study period was calculated for each patient.
Medication adherence was calculated using the medication refill adherence (MRA), which is considered the most appropriate method for this purpose due to its simplicity and efficacy, among the various methods available to calculate medication adherence using pharmacy computer records.6 This value was obtained by dividing the number of packs purchased by the number of study days for each patient. Given the varying number of pills per pack and the different posology of each DOAC, first the number of “total days' supply” from packs received at the pharmacy if the DOAC was taken correctly was calculated for each patient. This amount was then divided by the number of days each patient was included in the study, and finally multiplied by 100 to obtain the percentage adherence. In cases where more packs were purchased than were needed to adequately comply with the therapy during the period under assessment, adherence was rounded up to 100%. “Non-adherent” patients were those who had <90% treatment adherence.
Statistical analysisBaseline population characteristics were described as absolute number and percentage for categorical variables, and as mean and standard deviation or median and interquartile range (IQR) for continuous variables, as appropriate. Factors associated with “non-adherence” to DOACs in the univariate analysis (p<0.10) were included in a multivariate logistic regression analysis to identify independent predictors of non-adherence. Statistical analysis was performed with SPSS Statistics software version 21.0 (IBM Corp., Armonk, NY, USA). Values of p<0.05 (two-tailed) were considered statistically significant.
ResultsBaseline characteristicsThe baseline characteristics of the 264 patients included in the study are summarized in Table 1.
Baseline characteristics of the population.
| Characteristics | n=264 |
|---|---|
| Ages (years) | 74±12 |
| Male gender | 148 (56%) |
| Hypertension | 197 (75%) |
| Diabetes | 55 (21%) |
| Stroke/TIA/systemic thromboembolism | 42 (16%) |
| Stroke | 21 (12%) |
| Vascular diseasea | 77 (29%) |
| Congestive HF | 83 (13%) |
| Creatinine clearance (ml/min)b | 65 (47-86) |
| History of major bleedingc | 7 (3%) |
| Concomitant therapy with antiplatelets | 90 (34%) |
| CHA2DS2VASC | 3 (2-5) |
| HAS-BLED | 1 (1-2) |
TIA: transitory ischemic attack; heart failure.
Results are presented as mean ± standard deviation, number (%) or median (interquartile range).
Regarding anticoagulant therapy (Table 2), included patients were on DOAC therapy for a median of 439 days (IQR 269-638), corresponding to 14.4 months (IQR 9-30). About one-fifth (n=59, 22%) previously took VKA anticoagulation therapy before starting DOACs. Rivaroxaban was the most frequently prescribed DOAC, closely followed by apixaban. During the period under assessment, 51 patients (19%) took at least two different DOACs.
Characteristics of anticoagulant therapy.
| Anticoagulant therapy | n (%) |
|---|---|
| DOAC | |
| Rivaroxaban | 119 (45%) |
| Apixaban | 109 (41%) |
| Dabigatran | 62 (24%) |
| Edoxaban | 33 (13%) |
| Change of DOAC | 51 (19%) |
| Previous use of VKA | 59 (22%) |
VKA: vitamin K antagonist; DOAC: direct oral anticoagulant.
The results are presented as numbers (%).
In terms of payment, 89 patients were covered by the special system, and therefore had lower out-of-pocket expenses.
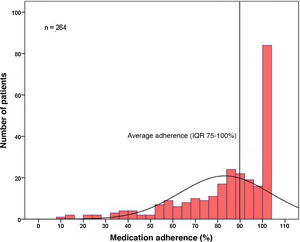
Medication adherenceThe included population adhered to DOAC therapy a median of 90% of the time (IQR 75-100%) (Figure 1). Only one-third of the patients (n=84, 32%) were fully compliant during the period under study (corresponding to 100% compliance). Analysis according to DOAC, median adherence was 91% (IQR 74-100%) for rivaroxaban, 87% (IQR 74-100%) for apixaban, 82% (IQR 48-100%) for dabigatran and 96% (IQR 83-100%) for edoxaban. There were no statistically significant differences between each of the DOACs (p=0.102) (Table 3).
Medication adherence – analysis according to direct oral anticoagulant.
| DOAC | Medication adherence | Non-adherent (<90%) | ||
|---|---|---|---|---|
| Rivaroxaban | 91% (74-100%) | p=0.102 | 48% | p=0.13 |
| Apixaban | 87% (74-100%) | 54% | ||
| Dabigatran | 82% (48-100%) | 57% | ||
| Edoxaban | 96% (83-100%) | 33% | ||
| Total | 90% (75-100%) | 51% | ||
DOAC: direct oral anticoagulant.
The results are presented as median (interquartile range).
A total of 134 patients (51%) were classified as “non-adherent” (adherence <90%), and the median therapeutic adherence in this group was significantly lower than that of patients classified as “adherent” 75% (IQR 57-84%) vs. 100% (IQR 97-100%), p<0.001). No statistically significant differences were found in the proportion of “non-adherent” patients to each of the DOACs (p=0.13) (Table 3).
Independent predictors of non-adherence were longer duration of therapy (adjusted odds ratio (OR) 1.06 for each month, 95% confidence interval (CI) 1.03-1.08, p<0.001), taking DOACs twice daily (adjusted OR 1.73, 95% CI 1.08-2.75, p=0.022) and payment according to the general system (adjusted OR 2.13, 95% CI 1.28-3.45, p=0.003) (Table 4). No other determinants of non-adherence were identified among the remaining clinical characteristics (gender, age, comorbidities, previous thromboembolic or hemorrhagic events, ischemic risk scores and hemorrhagic risk) or of anticoagulant therapy (previous VKA therapy or change of DOAC).
Predictors of medication non-adherence.
| Predictors of non-adherence | OR | CI 95% | p | Adjusted OR | CI 95% | p |
|---|---|---|---|---|---|---|
| Age | 0.99 | 0.97-1.01 | 0.41 | |||
| Gender (male) | 1.12 | 0.72-1.73 | 0.62 | |||
| Hypertension | 1.08 | 0.65-1.78 | 0.77 | |||
| Diabetes | 1.11 | 0.65-1.89 | 0.71 | |||
| Stroke/TIA/systemic thromboembolism | 0.88 | 0.64-1.14 | 0.30 | |||
| Stroke | 0.99 | 0.52-1.90 | 0.98 | |||
| Vascular diseasea | 0.88 | 0.54-1.42 | 0.60 | |||
| Congestive heart failure | 0.65 | 0.41-1.04 | 0.07 | |||
| Creatinine clearance (ml/min)b | 1.00 | 1.00-1.01 | 0.38 | |||
| History of major bleedingc | 1.33 | 0.29-6.05 | 0.71 | |||
| Concomitant therapy with antiplatelets | 0.96 | 0.61-1.53 | 0.88 | |||
| CHA2DS2VASC | 0.93 | 0.83-1.05 | 0.23 | |||
| HAS-BLED | 0.99 | 0.79-1.23 | 0.90 | |||
| Previous use of VKA | 0.86 | 0.50-1.46 | 0.57 | |||
| Twice daily dosing | 1.51 | 0.97-2.34 | 0.07 | 1.72 | 1.08-2.75 | 0.022 |
| Change of DOAC | 0.94 | 0.59-1.49 | 0.78 | |||
| Therapy duration under DOAC | 1.05 | 1.03-1.08 | <0.001 | 1.06 | 1.03-1.08 | <0.001 |
| Payment under general system | 1.72 | 1.08-2.74 | 0.02 | 2.13 | 1.28-3.45 | 0.003 |
CI: confidence interval; DOAC: direct oral anticoagulant; IQR: interquartile range; OR: odds ratio; TIA: transitory ischemic attack;. VKA: vitamin K antagonist.
Decreased adherence to therapy in chronic diseases is a well-known problem in clinical practice, and therapy with DOACs is no exception. Low adherence to anticoagulant therapy, rather than its ineffectiveness, has already been associated with the occurrence of ischemic strokes in patients chronically anticoagulated for AF in a Portuguese population.7 The aim of this study was not only to assess adherence to therapy in the context of AF, but also to clarify the impact of demographic, economic and clinical factors on compliance, identifying targets for possible future interventions in our population.
Medication adherenceIn this population, adherence to DOAC therapy was, on average, 90% during the period under study. However, only one-third of the patients achieved 100% adherence during the period under assessment, with the remaining two-thirds of the population unprotected for thromboembolic events over varying periods of time, as shown in Figure 1. Compared with other studies of DOACs,8,9 these results are slightly better than those reported; however, most available analyses do not report time on therapy but the percentage of compliant patients, after setting a value that marks adherence.
In this study, we considered “non-adherent” patients with a therapeutic adherence <90%. In many diseases, patients who comply properly with therapy more than 80% of the time are considered adherent.4 However, there is no universally defined value in the literature, varying according to the disease, assessment methods, and drugs in question. The value we chose took into account the severity of the events that DOAC aims to prevent and the practically immediate potential impact of treatment non-compliance in this context and considering their short half-life.
Using an adherence of <90% as a criterion to define “non adherence”, we found that, as in other chronic diseases, approximately 50% of patients were classified as non-compliant, a finding that has already been described in other reported studies of DOACs with the same cutoff point.10 The preventive nature of treatment with DOACs may explain this, since the lack of symptom alleviation associated with their use makes them particularly susceptible to non-compliance.11 Interestingly, even in patients in our population whose therapy was started as secondary prevention (16% with previous systemic thromboembolic events), there were no differences in adherence. Although the effect of noncompliance on ischemic or hemorrhagic events during the study period was not assessed, this association has been previously confirmed for several cardiovascular therapies,12 including DOACs.8
Predictors of non-adherenceIndependent predictors of non-adherence were longer duration of therapy, taking DOACs twice daily and payment under the general system for medication.
The association between longer duration of disease/treatment and lower medication adherence has been previously described in several settings,13 and our study confirms this impact also in the case of DOACs. The likelihood of non-compliance with the prescribed DOAC increased with longer therapy duration (6% higher probability of non-compliance for every month of treatment). Due to their ease of management, DOACs are likely to be introduced without close follow-up. These data reinforce the need for early reassessment, continuous monitoring, and education on how to take medication.
As for dosing regimen, the prescription of DOACs twice daily increased the probability of non-adherence almost twofold. Adherence is related to the number of doses taken daily for each drug,13 and an association between single-dose regimens and a greater likelihood of adherence to chronic medication in patients with non-valvular AF has been reported.14 A single daily dosing regimen is more convenient, and similar studies confirm greater adherence to DOACs with this type of dosing.15,16 Although the impact of different adherence between dosages with regards to efficacy and safety has not been assessed, previous studies show that single daily dosing appears to be as effective as twice daily dosing in preventing events such as stroke and venous thromboembolism.17 On the other hand, although single daily dosing may increase absolute adherence, twice daily regimens may be more “permissive” in terms of pharmacokinetic consequences in less compliant patients,18 a hypothesis based on pharmacological models but not yet confirmed in clinical trials.
Regarding cost, payment in the general regime was more than twice as likely to result in non-adherence. In special regime patients, the price of each package of DOAC is about half of the price paid by other patients,19 making it more affordable; this finding is in line with the empirical perception that the price of DOACs is still a barrier for our population. One solution to this problem would be to increase the co-payment of the general system, since, despite its high cost, the population-based cost-effectiveness studies conducted to date support the use of DOACs over VKAs20 Factors include the gains in years of quality of life and lower healthcare utilization based on the number of prevented events. We also hypothesize that the high price leads patients to “manage” their medication, omitting some doses to extend the duration of each pack and reduce monthly costs. Erroneous single daily dose rates of 27% and 30% have been reported for dabigatran and apixaban, respectively;21 our hypothesis may explain these data and justify the lower adherence to DOACs twice daily reported in this study.
Demographic factors such as age and gender did not influence adherence, nor did the presence of various comorbidities. A higher thromboembolic risk (based on the CHA2DS2VASC score) and taking VKA before the introduction of DOAC did not influence therapeutic compliance, although both have been previously associated with higher medication adherence.9,22
Comparison of DOACsNo significant differences were found in adherence to each of the four drugs (either in absolute terms or in classification as “non-adherent”), contrary to previous studies showing greater adherence to therapy with apixaban2 dabigatran9 or rivaroxaban.23,24 However, the unrepresentative sample of each one of these drugs meant we are not able to draw conclusions on comparisons between DOACs, and studies with larger populations, ideally randomized, are needed to enable comparisons of adherence (in addition to efficacy and safety). It should also be noted that the data presented were not adjusted for possible non-clinical confounding factors, such as the different dates of market introduction (giving, for example, advantage to edoxaban, which was introduced more recently and therefore hypothetically with shorter duration of therapy institution).
In patients whose DOAC was changed during the period under review, this alteration did not influence compliance, suggesting that whatever the reason for the change (adverse effect or not), it did not affect the way each patient adheres to this medication.
In summary, our results reveal insufficient medication adherence in the population under study and identify possible targets to improve compliance with DOAC therapy in AF, reminding us of the need to maintain regular follow-up of these patients. The impossibility of giving renewable prescriptions for this type of drugs may also be an obstacle to sustained adherence over time and may be another possible target for intervention. Due to the ease of introduction and management of DOACs compared to VKAs, the prevention of thromboembolic events in patients with non-valvular AF may now face its main challenge in the development of strategies for adequate adherence and medication persistence.
LimitationsThis study reports the results of a single tertiary center, and there may be differences in adherence and factors influencing compliance, not only in relation to other centers, but also in relation to consultations with other hospital specialties, and to patients followed in a primary health care setting.
Counting the number of packs collected from the pharmacy (necessary for the calculation of medication adherence), based on individual consultation of each patient's electronic prescription records, made this method time-consuming. This type of consultation also has the disadvantage of not including manual prescriptions or prescriptions issued outside the Portuguese National Health System (despite the attempts to minimize the impact of this type of prescription by excluding patients who had not had electronic prescriptions for at least six months). Automating these records and including all types of prescriptions and platforms in a common national database could be a useful clinical weapon to monitor adherence.
The adherence under study may have been underestimated due to periods of interruption of medication on medical advice (due to complications of therapy, elective procedures, or other reasons), as well as periods when the patient was hospitalized.
Finally, this was a retrospective analysis, with its inherent limitations. It was also a cross-sectional study, not allowing for the assessment of the trend in medication adherence at different points over the period under study.
ConclusionHalf of the patients (51%) with AF on DOAC were classified as non-adherent (compliance <90%). Longer duration of therapy, a.a twice daily dosing regimen and payment under the general system were independent predictors of non-adherence and may be targets for intervention to improve the compliance profile.
FundingThe authors declare that they did not receive any funding for the conduct of this study.
Conflict of interestJorge Ferreira received fees as a consultant and speaker from Boehringer-Ingelheim.
Please cite this article as: Brízido C, Ferreira AM, Lopes P, Strong C, Sá Mendes G, Fernandes Gama F, et al. Adesão à terapêutica com anticoagulantes diretos em doentes com fibrilhação auricular não valvular – uma análise de mundo real. Rev Port Cardiol. 2021;40:669–675.