Coronary artery anomalies (CAAs) are a rare entity but their true incidence in the general population has yet to be determined. Most CAAs are asymptomatic, but they are nevertheless the second leading cause of sudden death in apparently healthy young athletes.
The new imaging methods available to cardiologists, including CT angiography and MRI, now enable noninvasive diagnosis and characterization of these anomalies.
The authors review the literature and present a retrospective study of 360 consecutive patients who underwent cardiac CT angiography. Demographic, clinical and angiographic characteristics were studied. The incidence of CAAs in this population was 2.69%.
In order to better characterize this disorder, including diagnostic strategy, screening, treatment and prognosis, the authors suggest the establishment of a national registry of cardiac CT angiography. Such a registry would fill the existing gap in information on exams performed in the country, enriching current knowledge about this disease and noninvasive cardiac imaging in Portugal.
As anomalias das artérias coronárias (AAC) são uma entidade rara mas a sua verdadeira incidência na população em geral continua por definir. A maioria das AAC são assintomáticas, no entanto, constituem a segunda causa de morte súbita em jovens atletas, aparentemente saudáveis.
Os novos métodos de imagem ao dispor da Cardiologia, nomeadamente a AngioTC e a RM, permitem o diagnóstico e a caracterização não invasiva desta patologia.
Os autores fazem uma revisão da literatura e apresentam um estudo retrospectivo de 360 doentes consecutivos, submetidos a AngioTC cardíaca. Foram estudadas variáveis demográficas, clínicas e angiográficas. Nesta população a incidência de AAC foi de 2,69%.
Para melhor caracterização desta patologia, nomeadamente estratégia diagnóstica, de rastreio, terapêutica e prognóstico, os autores sugerem a realização de um Registo Nacional das AngioTC cardíacas. Este registo vai colmatar uma lacuna existente ao nível da informação dos exames realizados, enriquecendo o conhecimento actual sobre a patologia e a imagiologia cardíaca não invasiva em Portugal.
Coronary artery anomalies (CAAs) are congenital alterations in the origin, course or structure of the epicardial coronary arteries.
The true incidence of CAAs in the general population has yet to be determined, published series reporting very different percentages. Alexander and Griffith1 found an incidence of 0.3% in 1956, based on autopsy studies. In 1993, Cieslinski et al.2 reported an incidence of 0.97% in 4016 patients undergoing angiography between 1985 and 1989. However, these figures do not reflect the true incidence of CAAs in the general population, since autopsy studies are not performed as a matter of routine but for legal purposes, and angiography was performed in a selected group of patients in the latter study.
In 2002, Angelini3 found an incidence of around 1% in the general population, while a previous prospective analysis by the same author of 1950 patients undergoing cardiac computed tomography (CT) angiography reported an incidence of 5.64%,4 much higher than in previous studies.
By definition, CAAs occur in less than 1% of the general population,5 alterations with a higher incidence being considered variants of normal. No evidence has been found of differences in incidence according to gender or ethnicity.
Despite this uncertainty as to the true incidence of CAAs in the general population, there is evidence of higher incidence among young athletes and members of the armed forces who have suffered sudden cardiac death. In a registry of sudden death in athletes aged under 35 years, in whom cardiovascular disease was the demonstrated cause of death on autopsy, anomalous origin of a coronary artery in the opposite coronary sinus was responsible in 13% of cases, second only to hypertrophic cardiomyopathy.6 This illustrates the impact that strenuous physical activity can have on prognosis in these patients, and makes the true incidence of CAAs an issue of public health rather than of mere academic interest.
The authors review the literature and present a study on the incidence of CAAs in a population of patients undergoing cardiac CT angiography.
ClassificationThere is no consensus on the classification of CAAs. The principle behind the system proposed by Angelini7 (Table 1) is that the denomination of an artery is determined by the territory it supplies rather than by its origin or initial course. Thus, the right coronary artery (RCA) is the vessel that provides blood flow to the right ventricular (RV) free wall, the left anterior descending artery (LAD) provides blood flow to the anterior interventricular septum, and the circumflex artery (Cx) provides blood flow to the left ventricular (LV) free wall, on the obtuse margin of the heart.
Classification of coronary artery anomalies.
| A. Anomalies of origin and course |
| 1. Absent LMCA (split origin of LCA) |
| 2. Anomalous location of coronary ostium within aortic root or near proper aortic sinus of Valsalva for each artery |
| a. High |
| b. Low |
| c. Commissural |
| 3. Anomalous location of coronary ostium outside normal coronary aortic sinuses |
| a. Right posterior aortic sinus |
| b. Ascending aorta |
| c. Left ventricle |
| d. Right ventricle |
| e. Pulmonary artery |
| f. Aortic arch |
| g. Innominate artery |
| h. Right carotid artery |
| i. Internal mammary artery |
| j. Bronchial artery |
| k. Subclavian artery |
| l. Descending thoracic aorta |
| 4. Anomalous location of coronary ostium in improper sinus – variants |
| a. RCA arises from left coronary sinus, with anomalous course |
| i. Posterior atrioventricular groove or retrocardiac |
| ii. Retroaortic |
| iii. Between aorta and PA (intramural) |
| iv. Intraseptal |
| v. Anterior to pulmonary outflow tract or precardiac |
| vi. Posteroanterior interventricular groove |
| b. LAD arises from right anterior sinus, with anomalous course |
| i. Between aorta and PA (intramural) |
| ii. Intraseptal |
| iii. Anterior to pulmonary outflow tract or precardiac |
| iv. Posteroanterior interventricular groove |
| c. Cx arises from right coronary sinus, with anomalous course |
| i. Posterior atrioventricular groove |
| ii. Retroaortic |
| d. LMCA arises from right anterior sinus, with anomalous course |
| i. Posterior atrioventricular groove or retrocardiac |
| ii. Retroaortic |
| iii. Between aorta and PA |
| iv. Intraseptal |
| v. Anterior to pulmonary outflow tract or precardiac |
| vi. Posteroanterior interventricular groove from Angelini et al. 1999.4 |
| 5. Single coronary artery |
| B. Anomalies of intrinsic coronary arterial anatomy |
| 1. Congenital ostial stenosis or atresia (LMCA, LAD, RCA, Cx) |
| 2. Coronary ostial dimple |
| 3. Coronary aneurysm or ectasia |
| 4. Absent coronary artery |
| 5. Coronary hypoplasia |
| 6. Intramural coronary artery (muscular bridge) |
| 7. Subendocardial coronary course |
| 8. Coronary crossing |
| 9. Anomalous origin of posterior descending artery from the anterior descending branch or a septal penetrating branch |
| 10. Split RCA – variants |
| a. Proximal and distal posterior descending branches that both arise from the RCA |
| b. Proximal posterior descending branch that arises from the RCA, distal posterior descending branch that arises from the LAD |
| c. Parallel posterior descending branches×2 (arising from RCA, Cx) or “codominant” |
| 11. Split LAD – variants |
| a. LAD+first large septal branch |
| b. LAD, double (parallel LADs) |
| 12. Ectopic origin of first septal branch |
| a. RCA |
| b. Right sinus |
| c. Diagonal |
| d. Ramus |
| e. Cx |
| C. Anomalies of coronary termination |
| 1. Inadequate arteriolar/capillary ramifications |
| 2. Fistulas from RCA, LMCA or infundibular artery |
| D. Anomalous anastomotic vessels |
Cx: circumflex artery; LAD: left anterior descending artery; LMCA: left main coronary artery; PA: pulmonary artery; RCA: right coronary artery.
According to the same author, the following are considered normal features of the coronary anatomy: 2–4 ostia, located in the right and left coronary sinuses, with proximal orientation 45–90° to the wall of the aorta; the presence of a single common stem or trunk, the left main coronary artery (LMCA) (giving rise to the LAD and Cx); a direct proximal course, from ostium to destination; a subepicardial mid course, with adequate branches for the dependent myocardium, and terminating in capillaries.
Other authors have proposed classifying CAAs as severe, malignant or major versus minor, depending on their hemodynamic and clinical significance.8
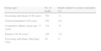
Regarding the incidence of the different anomalies, in the prospective analysis of 1950 patients undergoing CT angiography, Angelini4 found the incidences shown in Table 2.
Incidence of CAAs in a series of 1950 CT angiograms.
| n | % | |
| CAAs | 110 | 5.64 |
| Split RCA | 24 | 1.23 |
| Ectopic RCA (right cusp) | 22 | 1.13 |
| Ectopic RCA (left cusp) | 18 | 0.92 |
| Fistulas | 17 | 0.87 |
| Absent LCA | 13 | 0.67 |
| Cx arising from right cusp | 13 | 0.67 |
| LCA arising from right cusp | 3 | 0.15 |
| Low origin of RCA | 2 | 0.1 |
| Other anomalies | 3 | 0.27 |
CAAs: coronary artery anomalies; Cx: circumflex artery; LCA: left coronary artery; RCA: right coronary artery.
While most CAAs are asymptomatic, clinical presentation in adults as a result of myocardial ischemia can be in the form of angina, arrhythmias, syncope, infarction or sudden death. The latter is generally triggered by strenuous physical activity9 (Table 3).
Incidence of sudden death related to coronary artery anomalies.
| Group (age) | No. of deaths | Deaths related to coronary anomalies (%) |
| Exercising individuals (8–66 years) | 550 | 11 |
| General population (<40 years) | 162 | 0.6 |
| Competitive athletes (mean age: 17 years) | 134 | 23 |
| Runners (30–46 years) | 120 | 1.6 |
| Exercising individuals, Maryland, USA | 62 | 0 |
Of the various CAAs, origin of the LMCA in the right coronary sinus or of the RCA in the left sinus is associated with greater risk of sudden death.10,11 This is because such anomalies can lead to an acute angle in the artery's initial course or an interarterial course between the aorta and the pulmonary artery (PA), which dilate during exercise and compress the coronary artery.
CAAs can occur in isolation or be associated with other congenital disease, including transposition of the great vessels, tetralogy of Fallot and some forms of pulmonary atresia.12,13 In such cases, symptoms usually appear earlier and the diagnosis is made before adulthood.
To date no genetic mutations have been linked to CAAs. However, an article14 published in 2008 identified five cases of CAAs during screening of patients’ relatives; the authors highlight the need for further research in this area, given the malignant nature of some of these anomalies.
Diagnosis and screeningDiagnosing CAAs is a challenge, since patients are usually asymptomatic and physical examination reveals no abnormalities.
Given the fatal prognosis of certain CAAs, there is a need to develop screening methods, ideally noninvasive, as well as to define the target population.
As CAAs are the second leading cause of sudden death in young athletes, there is general agreement that a screening protocol should be established for young competitive athletes and those engaged in strenuous physical activity. The most appropriate diagnostic exams have yet to be defined.
Electrocardiogram/exercise testing/Holter monitoringThere are no specific electrocardiographic alterations that indicate a diagnosis of CAA. The presence of abnormalities suggestive of ischemia or cardiac arrhythmia in children or young adults can raise suspicion and prompt other diagnostic exams.
EchocardiographyEchocardiography is an attractive screening method since it is noninvasive, widely available, inexpensive and does not involve ionizing radiation. However, studies have reported variable sensitivity in diagnosing CAAs; the exam is dependent on the expertise of the operator, the age of the patient and the anomaly involved, transthoracic echocardiography gives better results in children than in adults, and the left coronary artery (LCA) is easier to identify than the RCA.15
Transesophageal echocardiography has greater sensitivity in detecting CAAs and can define their proximal course and flow pattern, but it is nevertheless a semi-invasive procedure.16
Invasive coronary angiographyConventional coronary angiography has been considered the gold standard method for diagnosing CAAs. However, it is an invasive exam and requires the use of nephrotoxic contrast agents and ionizing radiation. Furthermore, new imaging techniques, particularly cardiac CT angiography and magnetic resonance imaging (MRI), that provide three-dimensional assessment of the origin and course of arteries and their relationship to adjacent structures, have revealed certain shortcomings of invasive coronary angiography for the diagnosis of CAAs.17
Noninvasive coronary assessment by CT angiography and MRINoninvasive coronary assessment by CT angiography has evolved considerably in recent years. It is now a widely used method of assessing the coronary arteries, both for detecting atherosclerotic disease and identifying anomalies of origin or course, and several studies have demonstrated its accuracy for the latter purpose.18–20
Cardiac CT angiography provides better characterization of the origin of coronary arteries when selective characterization of the vessels is difficult, or even impossible, by invasive coronary angiography.21
The American Heart Association considers cardiac CT angiography an appropriate technique for diagnosis of CAAs, awarding it a score of 9, the maximum for a diagnostic method for a particular purpose.22
Recent advances in cardiac CT angiography have enabled more and better information to be obtained, while requiring ever smaller radiation doses, which overcomes an important disadvantage of its use compared to cardiac MRI.
Magnetic resonance coronary angiography is a noninvasive method that has advantages over cardiac CT angiography and conventional coronary angiography, namely that it does not require the use of nephrotoxic contrast agents or ionizing radiation.23 Its disadvantages are lengthy acquisition time and the fact that it is not widely available.
Both CT angiography and cardiac MRI require a degree of collaboration on the part of the patient, notably the ability to perform a breath-hold.
In the ACC/AHA 2008 guidelines for the management of adults with congenital heart disease24 cardiac CT angiography and cardiac MRI have a class I recommendation, level of evidence B, for the diagnosis of CAAs.
Given that CT angiography is more widely available than cardiac MRI and that it provides better definition than conventional angiography of the origin and course of coronary arteries and their relationship to other anatomical structures, it appears to be the method of choice in most cases of suspected CAA.
The initial approach in symptomatic patients should be a 12-lead electrocardiogram, exercise test and echocardiogram, which can suggest the diagnosis in some cases or reveal another cause of symptoms. Cardiac CT angiography should then be performed. Cardiac MRI, which has less potentially harmful effects, may have a role in screening asymptomatic individuals, particularly athletes.
TreatmentThe European Society of Cardiology guidelines for the management of grown-up congenital heart disease25 do not cover the treatment of patients with CAAs. In the ACC/AHA 2008 guidelines for the management of adults with congenital heart disease24 (Table 4), surgical revascularization is indicated in cases of the LMCA originating in the right coronary sinus and coursing between the aorta and PA (class I recommendation, level of evidence B) and of anomalies with an interarterial course with evidence of ischemia (class I recommendation, level of evidence B).
American College of Cardiology/American Heart Association recommendations.24
| Recommendation | Level of evidence |
| Class I | |
| 1. The evaluation of individuals who have survived unexplained aborted sudden cardiac death or with unexplained life-threatening arrhythmia, coronary ischemic symptoms, or left ventricular dysfunction should include assessment of coronary artery origins and course. | B |
| 2. CT or magnetic resonance angiography is useful as the initial screening method. | B |
| 3. Surgical coronary revascularization should be performed in patients with any of the following indications: | |
| a. Anomalous LMCA coursing between the Ao and PA. | B |
| b. Documented ischemia due to coronary compression (when coursing between the great arteries or in intramural fashion). | B |
| c. Anomalous origin of the RCA between the Ao and PA with evidence of ischemia. | B |
| Class IIa | |
| 1. Surgical coronary revascularization can be beneficial in the setting of documented vascular wall hypoplasia, coronary compression or obstruction to coronary flow, regardless of inability to document coronary ischemia. | C |
| 2. Delineation of potential mechanisms of flow restriction via intravascular ultrasound can be beneficial in patients with documented anomalous coronary artery origin from the opposite sinus. | C |
| Class IIb | |
| 1. Surgical coronary revascularization may be reasonable in patients with anomalous LAD coursing between the Ao and PA. | C |
Ao: aorta; LAD: left anterior descending artery; LMCA: left main coronary artery; PA: pulmonary artery; RCA: right coronary artery.
To assess the incidence of CAA in a population of patients undergoing cardiac CT angiography.
MethodsWe performed a retrospective study of 360 patients who underwent cardiac CT angiography in our institution between October 2009 and April 2011.
The exams were performed on a 64-slice scanner (Somatom Definition®, Siemens Medical Solutions).
Patients with heart rate of over 65bpm were medicated with 100mg oral metoprolol one hour before the exam, and all received sublingual nitroglycerin prior to image acquisition.
Demographic, clinical and angiographic characteristics were studied. CAAs were classified according to a modified version of the classification proposed by Angelini7 (intramyocardial courses not being considered anomalous). Significant coronary artery disease was defined as the presence of atherosclerotic plaques causing >50% stenosis.
ResultsOf the 360 patients undergoing cardiac CT angiography, 26 were excluded as images were not acquired, of whom 23 presented a calcium score of >1000 (indicating a high probability of the images being uninterpretable or of significant coronary artery disease) and three were unable to perform the breathhold.
Of the 334 patients assessed, 41% were male and mean age was 59±14 years.
Of this group, nine presented CAAs, an incidence of 2.69%. Four patients were male, and mean age was 61±11 years.
All patients were symptomatic (chest pain). The indications for cardiac CT angiography were inability to perform an exercise test in five patients, inconclusive ischemia test in two (one after myocardial perfusion scintigraphy and one after stress echocardiography), assessment of stent patency in one, and inability to selectively catheterize the coronary artery by conventional angiography in the other patient.
The most prevalent risk factors were hypertension (77.8%, seven patients) and dyslipidemia (55.6%, five patients).
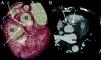
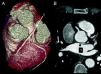
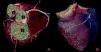
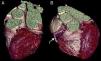
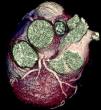
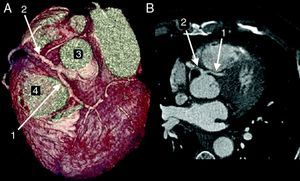
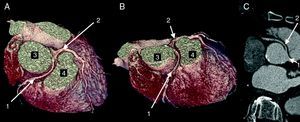
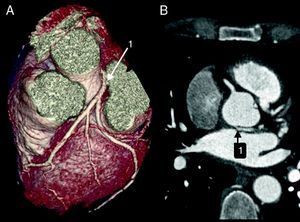
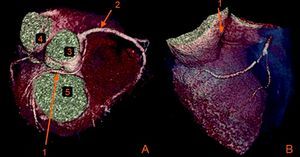
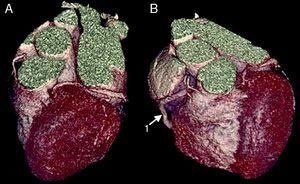
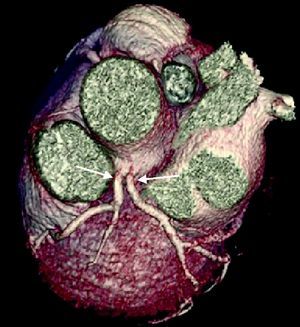
The CAAs diagnosed (Table 5) were: anomalous origin of the LCA in the right coronary sinus coursing between the aorta and PA (Figure 1); two cases of anomalous origin of the RCA in the left coronary sinus coursing between the aorta and PA (Figure 2); anomalous origin of the LCA in the non-coronary sinus (Figure 3); single RCA (Figure 5); anomalous origin of the RCA in the left coronary sinus (benign variant); anomalous origin of the Cx from the RCA coursing between the aorta and the left atrium (Figure 4); and two cases of separate ostia (Figure 6).
Coronary artery anomalies observed.
| Patient | Anomaly | Gender/age (years) | Other diagnoses |
| 1 | Anomalous origin of the LCA in the right coronary sinus coursing between the Ao and PA | M/53 | Significant CAD |
| 2 | Anomalous origin of the RCA in the left coronary sinus coursing between the Ao and PA | M/58 | HCMNon-significant CAD |
| 3 | Anomalous origin of the RCA in the left coronary sinus coursing between the Ao and PA | M/77 | Non-significant CAD |
| 4 | Single RCA | F/76 | No CAD |
| 5 | Anomalous origin of the Cx from the proximal RCA coursing between the Ao and the LA | M/58 | Non-significant CAD |
| 6 | Anomalous origin of the RCA in the left coronary sinus | F/59 | No CAD |
| 7 | Anomalous origin of the LCA in the non-coronary sinus | F/49 | No CAD |
| 8 | Separate ostia | F/47 | No CAD |
| 9 | Separate ostia | F/72 | Non-significant CAD |
Ao: aorta; CAD: coronary artery disease; Cx: circumflex artery; F: female; HCM: hypertrophic cardiomyopathy; LA: left atrium; LCA: left coronary artery; M: male; PA: pulmonary artery.
Four of these patients had non-significant CAD, and one had significant coronary disease.
All patients are currently under medical therapy; one (patient 1 – Table 5) is awaiting ischemia testing to guide therapeutic decision-making (surgical revascularization or angioplasty).
DiscussionWhen classifying CAAs, our group opted not to include intramyocardial courses, as various studies have reported that these occur in more than 1% of the population, and are thus a variant of normal. However, this definition is prior to 2002, and in the latest classification proposed by Angelini they are considered anomalies.
The incidence of CAAs in our selected patient group was 2.69%, higher than reported in the general population, although it should be borne in mind that incidence in the general population was also based on selected patient groups, namely those undergoing autopsy or invasive coronary angiography.
Two other Portuguese retrospective studies have been published to date, of 390626 and 366027 patients undergoing coronary angiography, which found CAA prevalences of 0.54% and 0.68%, respectively.
With regard to treatment, only patients 1, 2 and 3 (Table 5) had CAAs with surgical indication according to the guidelines. In the first case, besides anomalous origin of the LCA in the right coronary sinus coursing between the aorta and PA, a lesion causing 90% stenosis was also detected in the distal Cx. With a view to deciding the most appropriate treatment in this patient (surgical revascularization or angioplasty), stress echocardiography was performed to assess whether the ischemia causing the symptoms was in the territory of the LAD or of the Cx. However, the exam was inconclusive, and the patient is awaiting myocardial perfusion scintigraphy.
In the second case, the RCA had a subpulmonary course and so the patient was not considered for surgery; in addition, the patient's symptoms could also have been related to his hypertrophic cardiomyopathy.
The identification of CAAs associated with sudden death – anomalous origin of the LCA in the right coronary sinus coursing between the aorta and PA, and single RCA – in older adults (aged 77 and 76 years, respectively) makes these cases unusual; the fact that these patients had not previously presented any ischemic complications casts doubt on the malignancy of their anomalies, especially in the first case, which was not treated surgically.
Our series included two cases of separate ostia (absence of the LMCA). While normally not considered a CAA, it is included in the classification proposed by Angelini. If included, the incidence in our population was 2.69%, if excluded the incidence falls to 2.10%.
There is currently no national registry of cardiac CT angiography in Portugal. Such a registry could contribute to a better understanding of this and other heart diseases.
ConclusionsCAAs are rare and can be asymptomatic, but they are nevertheless the second leading cause of death in apparently healthy young athletes.
There is ongoing debate concerning their incidence, classification, screening, heritability and treatment.
The authors suggest the establishment of a national registry of cardiac CT angiography, which would contribute to a better understanding of this and other cardiac diseases and enrich current knowledge about noninvasive cardiac imaging in Portugal.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Dr. Pedro Gonçalves (Hospital da Luz) and Dr. Nuno Bettencourt (Centro Hospitalar de Vila Nova de Gaia) for their help with the cardiac CT angiographic studies.
Please cite this article as: Almeida, C; Anomalias das Artérias Coronárias. Rev Port Cardiol 2012;31(7-8):477-484.