Congenital absence of the pericardium is a very rare entity that is usually asymptomatic and hence difficult to diagnose. However, cases of sudden death have been reported in patients with partial pericardial defects (even asymptomatic ones), and such patients require surgical treatment.
We report the case of a 17‐year‐old patient with complete pericardial agenesis (diagnosed by chance during a cardiological consultation) and briefly review the radiological findings of this entity.
Agenesia congénita do pericárdio é uma entidade muito rara e de difícil diagnóstico, pois na maioria dos casos é geralmente assintomática. No entanto, tem havido relatos de morte súbita em pacientes com agenesia do pericárdio parcial, sendo necessário nestas situações a cirurgia (mesmo em pacientes assintomáticos).
Os objetivos deste trabalho são apresentar o caso de uma paciente de 17 anos com agenesia do pericárdio completo (diagnosticadas por acaso, durante uma visita ao cardiologista) e uma breve revisão dos achados radiológicos desta entidade.
Congenital defects of the pericardium are rare and in most cases asymptomatic.1–4 They used to be diagnosed as an incidental finding during surgery or autopsy, but the more widespread use of imaging techniques has increased their incidence.1–4 The most common congenital anomaly is complete absence of the left pericardium, which is usually asymptomatic.1 However, partial defects can cause symptoms due to ventricular herniation or entrapment and may even cause sudden death, and surgical repair is needed in these cases.1–4
We report the case of a 17‐year‐old woman attended in our institution for non‐specific but frequent symptoms, who was diagnosed with congenital complete absence of the pericardium.
Case reportWe report the case of a 17‐year‐old female patient with no relevant medical background, cardiovascular risk factors, or family history of heart disease or sudden death. The patient was referred to the cardiology department of our institution for episodes of dizziness lasting for seconds without spinning sensation, accompanied by cold sweats. Syncope was not present on any occasion and she did not report chest pain, palpitations or dyspnea. Physical examination and vital signs were all normal (blood pressure 102/63 mmHg and heart rate 74 bpm). Lower limbs showed no edema or signs of deep vein thrombosis, and femoral and pedal pulses were preserved. Diagnostic examinations revealed the following:
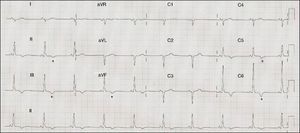
ECG: sinus rhythm at 71 bpm, PR interval 120 ms, right axis deviation, poor R‐wave progression in the precordial leads, inferolateral repolarization abnormalities and negative T waves in V5–V6, II, III, and aVF (Figure 1).
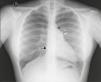
Chest X‐ray: displacement of the cardiac silhouette to the left without tracheal deviation, obliteration of the right cardiac border overlapping the spine and imprint of the pulmonary artery (Figure 2).
Figure 2.Posterior–anterior chest X‐ray showing levoposition of the heart without tracheal deviation, right heart border superimposed on the spine (straight arrow), imprint of the main pulmonary artery (curved arrow), interposition of lung parenchyma between the aortic arch and left pulmonary artery (asterisk) and flattening and elongation of the left ventricular contour (Snoopy sign).
Blood tests: no changes in the various parameters studied.
Holter ECG recording: sinus rhythm with extreme frequencies and circadian variation within normal range; no relevant ventricular or supraventricular arrhythmias, pauses or atrioventricular block.
Echocardiogram: apex offset to the left and posteriorly, particularly noticeable in modified parasternal long‐axis view. The left ventricle was non‐dilated with preserved systolic function, while the right ventricle was slightly dilated, especially in apical 4‐chamber view, forming the bulk of the ventricular apex. The left ventricular filling pattern was normal. The mitral valve opened correctly without a gradient, and tricuspid and aortic valve function was normal. There was mild tricuspid regurgitation with normal pulmonary artery systolic pressure. The inferior vena cava was dilated. The septum showed no alterations and the ascending aorta and aortic arch were normal. The pericardium could not be clearly identified.
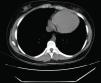
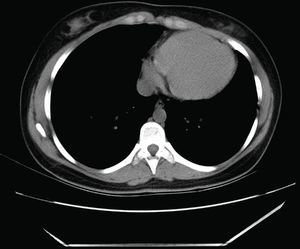
Computed tomography (CT): the cardiac silhouette was displaced, with the apex in left posterior position. The parietal pericardium could not be identified between epicardial and mediastinal fat (Figure 3).
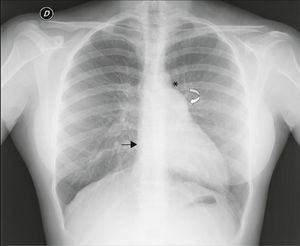
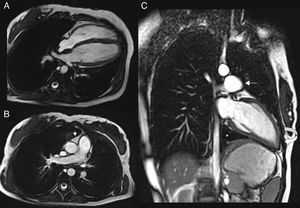
Cardiac magnetic resonance (CMR): the left heart was displaced, with flattening of the septum and the apex located posteriorly, pulmonary trunk protruding towards the left lung and interposition of pulmonary parenchyma between the diaphragm, descending aorta and diaphragmatic surface of the heart. Epicardial fat was in direct contact with the mediastinal pleura and the recess between the ascending aorta and pulmonary trunk was occupied by mediastinal fat. The pericardium could not be observed in any of the sequences (Figure 4).
Figure 4.(A and B) ECG‐gated cine gradient echo sequences, transverse plane. Left posterolateral rotation and displacement of the heart with flattening of the septum and apex in posterior location. Epicardial fat is in direct contact with the mediastinal pleura. The recess between the ascending aorta and the root of the pulmonary artery is filled with mediastinal fat (arrow); (C) ECG‐gated cine gradient echo sequences, coronal plane, showing levoposition of the heart with apex in posterior location (curved arrow), protrusion of the left pulmonary artery root (straight arrow) and interposition of lung parenchyma between the diaphragm, descending aorta and diaphragmatic surface of the heart (asterisk). The pericardium cannot be observed in any sequence.
All these findings were consistent with the diagnosis of complete pericardial agenesis, so we opted for clinical monitoring in outpatient cardiological consultations. The patient is asymptomatic at the present time.
DiscussionCongenital absence of the pericardium (CAP) is a rare entity that is difficult to diagnose and is usually identified incidentally in clinical autopsies or surgical procedures performed for other reasons.1–5 Its pathogenesis is multifactorial, but premature atrophy of the left common cardinal vein (left duct of Cuvier) during the 5th and 6th week of embryonic life compromises the blood supply to the left pleuropericardial fold, preventing it from closing normally.2,4,6
Its prevalence is approximately 0.002–0.004% in surgical and pathological series.2,7 It is three times more common in males and other congenital anomalies may be associated in 30–50% of cases, the most common being patent ductus arteriosus, atrial septal defect, mitral valve stenosis, bronchogenic cyst, tetralogy of Fallot, pulmonary sequestration, diaphragmatic hernia and pectus excavatum.2,4,8 CAP may also be found as a part of other disorders such as VATER association or Marfan or Pallister‐Killian syndromes.2,7
Six types of CAP have been described, including total absence, right‐sided defects (complete or partial), left‐sided defects (complete or partial), and diaphragmatic defects.2,8 The most common is complete left pericardial defect (70%), while right‐sided agenesis is found in 4%, diaphragmatic defects in 17% and total absence in 9%.4 Diaphragmatic pericardial agenesis is usually associated with absence of the left hemidiaphragm, in which there is a direct communication from the abdominal viscera to the heart. In 75% of cases of partial left agenesis there may also be a defect in the parietal pleura, resulting in herniation of lung parenchyma surrounding the adjacent vascular structures.4
CAP is usually asymptomatic, especially in complete absence of the pericardium.2,9 However, non‐specific chest pain, dyspnea, recurrent pulmonary infections, fatigue, angina, heart failure, pericarditis, arrhythmias, peripheral embolism, syncope and even sudden death have been reported.4,8
Diagnosis is often difficult because the physical examination is usually non‐specific, but it may reveal a significantly displaced apical impulse, basal ejection murmurs, apical mid‐systolic clicks and increased splitting of the second heart sound due to right bundle branch block.3,10,8,11,12 The ECG may be normal in small or partial defects, with sinus bradycardia induced by vagal stimulation the only finding. In other cases the ECG may show typical findings such as right axis deviation, incomplete or complete right bundle branch block and poor R‐wave progression due to clockwise rotation in the horizontal plane. Abnormalities of the ST segment and T wave are rare.3–7 Chest X‐ray findings suggestive of CAP include prominence of the main pulmonary artery, levoposition of the heart without tracheal deviation (which may be mistaken for cardiomegaly), absence of the right heart border because it is superimposed on the spine, and flattening and elongation of the left ventricular contour (Snoopy sign).3–8 As in our case, the echocardiogram may be helpful for the initial evaluation of CAP, with features related to abnormal cardiac position and movement: unusual echocardiographic windows, cardiac hypermobility, ‘teardrop’ appearance, paradoxical or flat systolic motion of the interventricular septum, severe tricuspid regurgitation and right ventricular dilatation. However, echocardiography is usually less useful in partial defects.4,13
CT can reveal the abnormal rotation and displacement of the heart and it may also show absence of the parietal pericardium as direct contact between mediastinal and epicardial fat. Nevertheless, absence of pericardium is difficult to identify in left‐sided or posterior defects, due to the smaller quantity of fat in this location, which is the main limitation of this imaging modality.4,9
CMR is considered the gold standard imaging technique due to its better soft tissue definition using spin‐echo sequences synchronized with the cardiac cycle and its ability to detect focal myocardial infarctions.3,14 When the pericardium is not visualized directly, there may be indirect CMR signs that distinguish between partial left agenesis (prominence of the main pulmonary artery with normal heart position) and total left agenesis (levoposition of the heart, contact between the left atrium and descending aorta, and the preaortic recess filled with mediastinal fat). In addition, CMR can diagnose cardiac herniation due to a partial defect; annular constriction of the apical ventricular myocardium is the most common cause of death in these patients.2–18 Treatment is not required in total agenesis or complete unilateral agenesis,2,3,6,10,8,11,12 although one case has been reported in which a complete left‐sided defect required surgical correction to alleviate symptoms.19 Partial defects should be surgically repaired in symptomatic patients and in asymptomatic patients with signs of ventricular strangulation diagnosed by an imaging technique.4 There is controversy concerning asymptomatic patients with atrial herniation, since no cases of sudden death have been reported for this reason.4,15
In conclusion, CAP is an unusual anomaly and usually asymptomatic. Nevertheless, it is important to diagnose and characterize this entity accurately due to the risk of complications and sudden death in patients with partial defects. Surgical treatment is reserved for symptomatic patients and in asymptomatic patients at risk for ventricular entrapment.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.