P-wave dispersion (PWD) and cardiac troponin levels are independently associated with the recurrence of atrial fibrillation (AF) in patients with paroxysmal AF (PAF). We investigated the clinical usefulness of combining PWD and cardiac troponin I to predict AF recurrence in patients presenting to the emergency department with PAF.
MethodsThis study included 65 patients with PAF who were divided into three groups according to baseline troponin I and PWD values (group 1, troponin I<0.11 ng/dl and PWD<44.5 ms; group II, troponin I<0.11 ng/dl and PWD≥44.5 ms, or troponin I≥0.11 ng/dl and PWD<44.5 ms; group III, troponin I≥0.11 ng/dl and PWD≥44.5 ms).
ResultsThe AF recurrence rate was significantly higher in group III than in groups I and II. Multivariate analysis revealed that the troponin I and PWD values in group III (odds ratio: 7.236, 95% confidence interval: 1.879-27.861, p=0.004) were independent predictors of AF recurrence.
ConclusionsThe combined use of PWD and basal troponin I levels is a better predictor of AF recurrence than either value alone.
A dispersão da onda P (DOP) e os níveis séricos de troponina cardíaca estão independentemente associados à recidiva de fibrilhação auricular (FA) em doentes com FA paroxística (FAP). Estudámos a utilidade clínica da combinação entre a DOP e a troponina cardíaca I para prever a recidiva de FA em doentes que se apresentaram no serviço de urgência com FAP.
MétodosEste estudo incluiu 65 doentes com FAP, divididos em três grupos de acordo com a troponina I basal e com os valores da DOP (grupo 1, <0,11 ng/dL e DOP<44,5 ms; grupo II, troponina I<0,11 ng/dL e DOP≥44,5 ms, ou troponina I≥0,11 ng/dL e DOP<44,5 ms; grupo III, troponina I≥0,11ng/dL e DOP≥44,5 ms).
ResultadosA recorrência de FA foi significativamente mais elevada no grupo III do que nos grupos I e II. A análise multivariada revelou que a troponina I e os valores da DOP no grupo III (odds ratio: 7,236, intervalo de confiança 95%: 1,879-27,861, p=0,004) foram preditores independentes da recidiva de FA.
ConclusõesA utilização combinada da DOP e dos níveis de troponina I basal constitui um melhor preditor de recidiva de FA do que os dois valores isolados.
Atrial fibrillation (AF) is the most common type of arrhythmia. It affects millions of people worldwide, and its prevalence increases with age.1 AF is associated with stroke and other thromboembolic events, low exercise capacity, impaired quality of life, heart failure, left ventricular dysfunction, and increased mortality.2 In addition to aging, various clinical conditions are associated with AF, including hypertension, diabetes, coronary artery disease, valvular heart disease, cardiomyopathies, symptomatic heart failure, atrial septal and other congenital heart defects, obesity, thyroid dysfunction, chronic obstructive pulmonary disease, sleep apnea, and chronic kidney disease. Screening for predictors of AF in at-risk groups plays a key role in preventing the condition.2
Previous studies have established the clinical value of left atrial size and premature atrial contraction, based on Holter recordings and echocardiography, as predictors of AF.3,4 Additionally, maximum P-wave duration (Pmax) and P-wave dispersion (PWD) have been used to assess the risk of AF.5–8 PWD is defined as the difference between Pmax and minimum P-wave duration (Pmin) recorded by 12-lead surface electrocardiography (ECG).6 Measurement of PWD is an easy-to-use, low-cost method that reflects atrial muscle activation, left atrial size, and changes in electrical conduction in the atrium.7
Cardiac troponin I is a specific and sensitive marker of cardiac muscle damage. Recent studies have shown that elevated levels of high-sensitivity cardiac troponin T (hs-TnT) are associated with poor cardiovascular outcomes in patients with AF.9–11 Furthermore, a recent study found that elevated hs-TnT was associated with AF recurrence in patients with AF independently of traditional risk factors and left atrial size.12
Although PWD and cardiac troponin have been reported to independently predict AF recurrence, to our knowledge no study has assessed the usefulness of combined PWD and cardiac troponin I values for predicting recurrent AF in patients with paroxysmal AF (PAF). We therefore investigated the clinical value of the combined use of PWD and cardiac troponin I values for predicting AF recurrence in patients presenting to the emergency department with PAF.
MethodsThis cross-sectional case-control study included 110 patients who presented to the emergency department with palpitations within the preceding 48 hours between April 2017 and December 2018. Patients presenting with sinus tachycardia (n=10), supraventricular tachycardia (n=7), ventricular tachycardia (n=3), ventricular extrasystoles (n=8), multifocal atrial tachycardia (n=5), coronary artery disease (n=5), and other conditions (n=7) were excluded; thus, 65 patients with PAF were finally included. The study was approved by the local ethics committee, and all participants provided informed consent. All patients underwent routine 12-lead ECG and blood tests on admission to the emergency department. Patients with no history of chronic AF were diagnosed with PAF based on ECG findings. All patients were examined by a cardiologist in the emergency department and appropriate medical interventions and follow-up procedures were performed. Normal sinus rhythm was restored in all patients through medical management without the need for electrical therapy.
Cardiac troponin I concentrations and routine blood parameters were measured in blood samples collected at admission. PWD, defined as the difference between the longest and shortest P-waves on 12-lead ECG, was recorded. Two independent clinicians measured PWD, and the inter- and intraobserver agreement rates were 98.5% and 99%, respectively. All patients were closely monitored for six months for recurrent AF. Patients with recurrent arrhythmias were re-examined in the emergency department and underwent 12-lead surface ECG to assess cardiac rhythm. Blood samples were taken to measure troponin I levels. The presence of AF on ECG was taken to indicate recurrent PAF.
Patients were divided into three groups according to baseline troponin I and PWD values (group 1, troponin I 0.11 ng/dl and PWD 44.5 ms; group II, troponin I 0.11 ng/dl and PWD≥44.5 ms, or troponin I≥0.11 ng/dl and PWD 44.5 ms; group III, troponin I≥0.11 ng/dl and PWD≥44.5 ms).
Electrocardiographic analysisAfter a rest period of 20 min in the supine position, 12-lead ECG was performed at a speed of 50 mm/s and voltage of 20 mV/mm in all patients. P-wave duration was manually calculated simultaneously. Three QRS complexes were recorded for each lead. P-wave onset was defined as the time of the first visible upward/downward deviation from baseline for positive/negative waves, and offset was defined as the return of the waveform to baseline. Pmax, measured in any of the 12 leads of the surface electrocardiogram, was taken as the longest atrial conduction time. PWD was defined as the difference between Pmax and Pmin (PWD=Pmax–Pmin).
Statistical analysisAll statistical analyses were performed using IBM SPSS for Windows version 22.0 (IBM SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as means ± standard deviation or medians (25-75% interquartile range) and categorical variables are expressed as percentages. The Shapiro-Wilk test was used to assess the normality of the data. Between-group comparisons of continuous variables were performed using the Student's t test for parametric variables and the Mann-Whitney U test for nonparametric variables. The chi-square test was used to compare categorical variables. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off values for PWD and baseline troponin I. Multivariate logistic regression analysis was performed to identify independent predictors of recurrent PAF. A p-value <0.05 was considered to indicate statistical significance.
ResultsThe study included 65 patients with PAF. Patients were classified as having recurrent or non-recurrent AF. AF recurred in 21 (32.3%) patients within six months. Selected descriptive and clinical characteristics of the groups are shown in Table 1 according to AF recurrence status. Clinical characteristics and laboratory findings were not significantly different between the recurrent and non-recurrent AF groups (Table 2).
Selected descriptive and clinical features in recurrent and non-recurrent atrial fibrillation groups.
| Recurrent AF group (n=21) | Non-recurrent AF group (n=44) | p | |
|---|---|---|---|
| Age, years | 47.1±5.0 | 46.1±5.8 | 0.568 |
| Male gender, % | 12 (57.1) | 26 (59.1) | 0.882 |
| BMI, kg/m2 | 28.1±5.0 | 26.4±4.4 | 0.163 |
| SBP, mmHg | 127.1±10.1 | 122.3±12.9 | 0.182 |
| DBP, mmHg | 77.1±9.0 | 76.8±7.2 | 0.690 |
| Heart rate, bpm | 64.5±7.6 | 64.0±8.3 | 0.657 |
| Ejection fraction, % | 56.2±3.4 | 57.9±3.8 | 0.511 |
| Left atrium, mm | 33.4±2.5 | 32.8±3.3 | 0.849 |
| Medical therapy | |||
| Beta-blocker, n (%) | 4 (19) | 21 (48) | 0.056 |
| Calcium channel blocker, n (%) | 4 (19) | 16 (36) | |
| Propafenone, n (%) | 13 (62) | 7 (16) | |
AF: atrial fibrillation; BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure.
Baseline laboratory characteristics in recurrent and non-recurrent atrial fibrillation groups.
| Blood glucose, mg/dl | 91.9±5.5 | 89.3±6.5 | 0.113 |
| TC, mg/dl | 168.8±70.9 | 181.1±49.5 | 0.421 |
| LDL, mg/dl | 113.1±41.5 | 125.7±41.2 | 0.254 |
| HDL, mg/dl | 47.1±18.8 | 44.8±15.3 | 0.752 |
| Urea, mg/dl | 39.7±19.0 | 34.3±13.3 | 0.523 |
| Creatinine, mg/dl | 1.02±0.32 | 0.88±0.22 | 0.163 |
| ESR, nm/h | 18.0±12.9 | 21.2±17.8 | 0.822 |
| CRP, mg/l | 5.4±5.6 | 4.9±7.6 | 0.066 |
| Na, mmol/l | 136.3±2.9 | 137.6±3.1 | 0.148 |
| K, mmol/l | 4.0±0.7 | 4.1±0.5 | 0.436 |
| Hemoglobin, g/dl | 13.8±1.7 | 14.0±1.8 | 0.630 |
| WBC, 103/mm3 | 11.5±3.2 | 11.4±3.4 | 0.828 |
| Platelets, 103/mm3 | 246.0±63.2 | 243.6±67 | 0.817 |
CRP: C-reactive protein; ESR: erythrocyte sedimentation rate, HDL: high-density lipoprotein, LDL: low-density lipoprotein; TC: total cholesterol; WBC; white blood count.
P-wave dispersion and baseline troponin I values are shown in Table 3. PWD (p=0.001) and baseline troponin I levels (p=0.004) were significantly higher in patients with AF recurrence than in those without recurrence. Furthermore, the percentage of troponin I-positive patients in the recurrent AF group was significantly higher than in the non-recurrent group.
Distribution of P-wave dispersion and troponin I values between recurrent and non-recurrent atrial fibrillation groups.
| Recurrent AF group (n=21) | Non-recurrent AF group (n=44) | p | |
|---|---|---|---|
| PWD, ms | 51.9±14.2 | 35.8±9.0 (16-50) | <0.001 |
| Troponin I, ng/ml | 0.16±0.16 | 0.07±0.11 | 0.009 |
| Troponin categorya | |||
| Positive, % | 14 (66.7) | 17 (38.6) | 0.034 |
| Negative, % | 7 (33.3) | 27 (61.4) | |
AF: atrial fibrillation; PWD: P-wave dispersion.
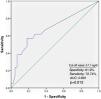
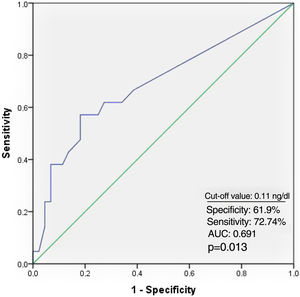
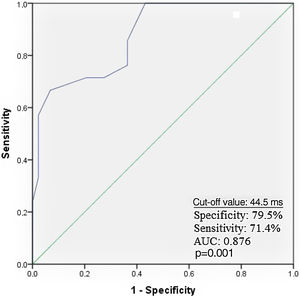
Receiver operating characteristic curve analysis curve analysis was performed to determine the optimal troponin I and PWD cut-off values for predicting AF recurrence. Troponin I≥0.11 ng/ml predicted AF recurrence with a specificity of 61.9% and sensitivity of 72.7% (Figure 1). PWD≥44.5 ms predicted AF recurrence with a specificity of 79.5% and sensitivity of 71.4% (Figure 2).
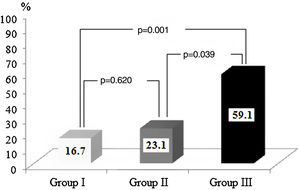
In total, 22 (%) patients were included in group III (troponin I≥0.11 ng/ml and PWD≥44.5 ms, 13 (20%) were included in group II (troponin I≥0.11 ng/ml or PWD≥44.5 ms), and 30 (46.2%) were included in group I (troponin I 0.11 ng/ml and PWD 44.5 ms). The rate of AF recurrence was significantly higher in group III than in group I (59.1% vs. 16.7%, p=0.001) and group II (59.1% vs. 23.1%, p=0.039) (Figure 3).
Multivariate logistic regression analysis revealed that the troponin I and PWD values in group II (odds ratio [OR]: 1.025, 95% confidence interval (CI): 0.189-5.553, p=0.977) and group III (OR:7.236, 95% CI: 1.879-27.861, p=0.004) were independent predictors of AF recurrence (Table 4).
Multivariate logistic regression analysis showing independent predictors of recurrence of atrial fibrillation.
| Variable | OR (95% CI) | p |
|---|---|---|
| Blood glucose | 1.106 (0.990-1.235) | 0.076 |
| Group Ia | - | - |
| Group II | 1.025 (0.189-5.553) | 0.977 |
| Group III | 7.236 (1.879-27.861) | 0.004 |
Entered variables: age; C-reactive protein; white blood count; erythrocyte sedimentation rate; glucose; platelets; gender; groups.
CI: confidence interval; OR: odds ratio.
We investigated the clinical value of the combined use of troponin I levels and PWD for predicting AF recurrence in patients with PAF. We found that the combined use of troponin I and PWD was superior for predicting AF recurrence than either measure alone. To our knowledge, our study is the first to demonstrate the clinical importance of the combined use of troponin I and PWD for predicting AF recurrence in patients with PAF.
Because AF is associated with thromboembolic events and various cardiac and systemic conditions that affect quality of life, treatment and prevention of complications are essential. Thus, predicting the recurrence of AF in patients with PAF is important. Several parameters that can be obtained non-invasively have been used to predict AF, including left atrial size, Holter recordings, and premature atrial contractions,3–5 as well as ECG and laboratory parameters.
Prolonged intra- and interatrial conduction times and non-homogeneous propagation of sinus impulses are characteristic of PAF. An increase in PWD indicates heterogeneous intra- and interatrial conduction.13 Previous studies have shown that PWD is significantly greater in patients with PAF than in controls, and that PWD≥40 ms can differentiate patients with PAF with 83% sensitivity and 85% specificity.7,9,13–16 Moreover, atrial diameter has a significant effect on P-wave duration and PWD, while reverse atrial electrical remodeling has been shown to reduce both parameters.14,17 Our finding that PWD was significantly greater in the recurrent AF than in the non-recurrent AF group is consistent with previous studies. Taken together, our findings suggest that PWD is an independent predictor of AF recurrence.
Cardiac troponin I is a sensitive and specific marker of cardiac muscle damage. Several studies have shown that increased serum cardiac troponin T levels are associated with poor cardiovascular outcomes and AF recurrence in patients with AF.10–12,18 Our finding that troponin I levels were markedly higher in patients with than without AF recurrence is consistent with previous findings. Furthermore, we classified troponin I values as positive or negative based on our laboratory reference range and found that troponin I positivity was markedly higher in the recurrent AF than in the non-recurrent AF group. These findings suggest that elevated cardiac troponin I levels and positivity are independent predictors of AF recurrence.
Previous studies found that the combination of two parameters has higher predictive value that either parameter alone.19–21 Although PWD and troponin I levels are independently associated with increased risk of AF recurrence, the clinical importance of the combined use of these values to predict AF recurrence in patients with PAF has not been investigated. We found that the rate of AF recurrence was significantly higher in patients in group III than in those in groups I and II. Moreover, multivariate regression analysis revealed that the patients in group III had a roughly 2.5-fold higher rate of AF recurrence than those in group II, and that the OR of group III (7.236) was higher than that of group II (1.025). Our findings suggest that the combined use of PWD and baseline troponin I values has higher predictive value than PWD or troponin alone.
LimitationsOur study had some limitations. First, the sample size was small. Second, we measured only baseline troponin I values and did not perform serial troponin I measurements. Third, only patients with PAF were included in the study. Comparison of PAF and persistent AF patients with healthy individuals could have provided greater insight. Further prospective studies with larger samples are needed to better understand the clinical value of the combined use of PWD and troponin I levels.
ConclusionPWD is a simple and easily measured ECG marker, and troponin I can be obtained quickly and inexpensively during routine laboratory analysis. In this study, we show for the first time that the combined use of PWD and basal troponin I levels is a better predictor of AF recurrence than either value alone.
Conflicts of interestThe authors have no conflicts of interest to declare.