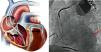
Cardiac resynchronization therapy (CRT) is an electrical device treatment that allows the heart to beat in a more coordinated and synchronized manner, thus improving the electrical dyssynchrony found in many patients with heart failure (HF) (Figure 1) and increasing left ventricular (LV) filling time and reducing mitral regurgitation and ventricular asynchrony.1 It is indicated in selected patients with severe LV dysfunction and a broad QRS complex, with significant benefits demonstrated in cardiac output and hemodynamic parameters, and is associated with reverse remodeling and improvements in functional capacity and quality of life, as well as reductions in clinical symptoms, hospitalization due to HF, and mortality.2,3 CRT can be achieved with a device designed only for pacing (CRT-P) or can be incorporated into a combination device with an implantable cardioverter-defibrillator (CRT-D).
Unfortunately, despite appropriate selection criteria, a variable proportion of eligible patients, known as non-responders, fail to benefit from this treatment. A number of reasons for this failure have been postulated, and suboptimal atrioventricular (AV) intervals are considered to be a major factor in this complex problem.4 Suboptimal LV filling time or LV dyssynchrony persisting after CRT may reduce the benefits of this therapy. In a subset of patients with LV dysfunction there is a disturbance in coordination of atrial and ventricular activation causing AV dyssynchrony. Furthermore, there is a spectrum of ventricular conduction abnormalities varying from a proximal barrier to a more diffuse slowing of conduction. As a consequence, the LV is electrically activated throughout myocardial tissue, leading to mechanical interventricular and intraventricular dyssynchrony.5 CRT devices enable manipulation of AV and interventricular (VV) timings in order to maximize LV performance. Previous studies have shown that significant improvements in hemodynamic function can be obtained by optimizing device programming.4,6 However, AV delay optimization is often poorly performed in clinical CRT practice, and is frequently programmed empirically,7 for reasons of time, cost, and complexity. However, there are also several other questions regarding the systematic optimization of AV and VV intervals, particularly the best method to perform optimization, when and how often, and its impact on daily practice.
The incremental value of optimization over empirical device programming was evaluated in a meta-analysis of combined data on a total of 4356 patients with HF treated with CRT.8 According to this analysis, routine AV and/or VV delay optimization has a neutral effect on clinical or echocardiography outcomes, making it even more controversial to perform in all patients undergoing CRT. A recent large long-term follow-up study from Japan, presented during the American College of Cardiology 2014 Scientific Sessions, showed that AV interval optimization significantly improved survival in patients with CRT, whereas VV interval optimization showed no clinical benefit.9 According to the 2013 European Society of Cardiology guidelines on cardiac pacing and cardiac resynchronization therapy, current literature does not support AV and VV optimization routinely in all patients receiving CRT.10 However, in non-responders and in those with ischemic heart disease or in need of atrial pacing, assessment of AV and VV delay may be recommended in order to correct suboptimal device settings.10
Theoretically, a system that continually adjusts AV and VV delays according to the patient's daily activities, hemodynamic variations, or medications would be an ideal option. Should programming settings be periodically reviewed? And what if an automatically effective algorithm could be incorporated into devices to improve CRT response?
At present, the automatic methods available for AV and VV interval optimization include those based on intracardiac electrogram (IEGM) measurements (QuickOpt™, SmartDelay™ and AdaptivCRT®) and the peak endocardial acceleration (SonR) sensor, a micro accelerometer built into the tip of the right atrial lead that detects cardiac muscle vibrations reflecting the first heart sound. This sensor provides a signal amplitude measurement that correlates with LV dP/dtmax (reflecting LV contractility). If the difference between the new area measured by the sensor compared to the previous week is ≥10% the new configuration settings for AV and/or VV will be applied. The SonR sensor is programmed to perform weekly measurements and automatic optimizations during rest and also under effort, if the patient's heart rate exceeds 90 bpm.
IEGM- and hemodynamic device-based algorithms are considered safe methodologies, with randomized clinical studies showing interesting data regarding their benefits in clinical and echocardiographic outcomes. The Freedom trial assessed the safety and efficacy of frequent CRT optimization (3, 6, 9, and 12 months) using the QuickOpt method.11 The study showed non-inferiority compared to standard of care (no optimization or a single echo-based optimization within the first four weeks after implant) regarding clinical outcome during the first year after CRT. In the Smart AV trial, the SmartDelay group results, including NYHA class, quality of life and echocardiographic parameters, were equivalent to the echo-optimized and the empirical programming groups.12 The Adaptive CRT trial also had non-inferior clinical results regarding safety and effectiveness compared to echo-based optimization in the first six months of follow-up,13 as well as showing a significantly lower risk of atrial fibrillation compared to conventional biventricular therapy.14 Finally, the Clinical Evaluation on Advanced Resynchronization (CLEAR) study, a randomized pilot study of optimization of CRT in sinus rhythm patients using the SonR sensor algorithm, with a population of 199 patients, showed superiority compared to standard of care regarding clinical outcome (mostly driven from NYHA class) after one year of follow-up.15 Recently, a retrospective analysis of this study, regarding the association between the frequency of AV delay and VV delay optimization and one-year outcomes, concluded that systematic CRT optimization (at implant and at three and six months) was associated with a higher percentage of improved patients, fewer deaths and fewer hospitalizations,16 emphasizing the potential clinical benefit of frequent CRT optimization programming on long-term clinical response in this population.
Interest in the use of automatic algorithms to improve cardiac function and hemodynamics in patients undergoing CRT is increasing. Multicenter prospective randomized trials are underway to test the superiority of automatic IEGM- and hemodynamic-based algorithms compared to standard in-office manual echocardiography device optimization to improve CRT response in clinical practice.