Transcatheter aortic valve replacement (TAVR) is an increasingly common procedure for the treatment of aortic stenosis in elderly patients with comorbidities that prevent the use of standard surgery. It has been shown that implantation without aortic regurgitation is related to lower mortality. Mild paravalvular regurgitation is inevitable in some cases due to calcification of the aortic annulus and its usually somewhat elliptical shape. Central regurgitation is less common, but has been associated with valve overdilatation in cases in which reduction of paravalvular regurgitation was attempted after the initial inflation. However, there are no reported cases of central prosthetic aortic regurgitation due to acute LV dysfunction. We report a case in which central aortic regurgitation occurred due to transient ventricular dysfunction secondary to occlusion of the right coronary artery by an embolus. The regurgitation disappeared after thrombus aspiration and normal ventricular function was immediately recovered.
A substituição percutânea de válvula aórtica (transcatheter aortic valve replacement – TAVR) tem-se tornado cada vez mais frequente para o tratamento da estenose aórtica em pacientes idosos com doenças associadas, para evitar a cirurgia convencional. Sabe-se que, não havendo refluxo aórtico após o implante, a mortalidade será mais baixa. Em alguns casos, contudo, um refluxo paravalvular moderado é inevitável, devido à calcificação do ânulo-aórtico e à sua configuração geralmente elíptica. O refluxo central é mais raro, tendo sido associado à excessiva dilatação da válvula em casos em que se tentou redução do refluxo paravalvular, após a inflação inicial. Entretanto, não foram referidos casos de refluxo aórtico central por disfunção ventricular esquerda aguda. Referimos um caso em que ocorreu refluxo aórtico central por disfunção ventricular transitória, secundária à oclusão da artéria coronária direita por êmbolo. O refluxo desapareceu após aspiração do trombo, sendo recuperada imediatamente a função ventricular normal.
Paravalvular aortic regurgitation (PAR) is relatively common after transcatheter aortic valve replacement (TAVR). In a recent meta-analysis, the incidence of moderate or severe PAR was 11.7%, although it is apparently less frequent with the Edwards SAPIEN valve (Edwards Lifesciences; Irvine, CA, USA) than with the CoreValve® (Medtronic; Tolochenaz, Switzerland) (9.1% vs. 16.0%, respectively; p=0.005). The PARTNER study reported severe PAR in 6.8% of cases, compared to 1.6% found in the meta-analysis by Athapan et al., while moderate PAR occurred in 10.5% of cases in both studies.1,2 Minimal PAR was observed in 46% according to the meta-analysis. Three factors have been identified as predictors of PAR after TAVR: prosthetic valve undersizing, improper implantation depth and aortic valve calcification. The importance of this issue is that moderate or severe regurgitation has been associated with increased mortality at both 30 days and one year; mild regurgitation has been linked to higher mortality, although this is only a tendency in some studies.1–4 On the basis of current data, some reports have emphasized the importance of selecting the most appropriate prosthesis size using imaging techniques such as computed tomography.5
However, central aortic regurgitation related to TAVR has not often been reported, as it is usually associated with overdilatation after implantation when the valve is too small or when reducing PAR. To our knowledge, central prosthetic aortic regurgitation due to acute left ventricular (LV) dysfunction has not been described, and should be borne in mind in perioperative echocardiography, to identify a possible reversible cause of this phenomenon. We present a case reflecting these issues.
Case reportWe present the case of an 82-year-old hypertensive woman. Five years previously, percutaneous transluminal coronary angioplasty had been performed due to occlusion of the mid left anterior descending artery (LAD), and two Cypher stents, 3/33 mm and 2.5/28 mm, were implanted. In the previous year she had symptoms of effort angina and grade 3 dyspnea. The LAD stents were in good condition and no other lesions were observed. Severe aortic stenosis was observed with aortic valve area 0.75 cm2, peak gradient 80 mmHg and normal LV function with severe hypertrophy. The EuroSCORE for this patient was 20%.
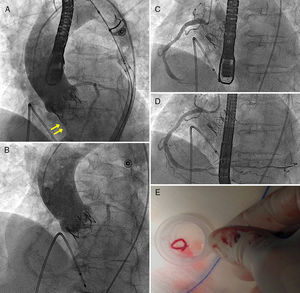
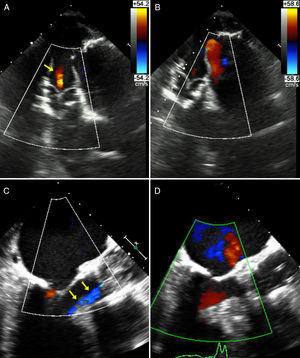
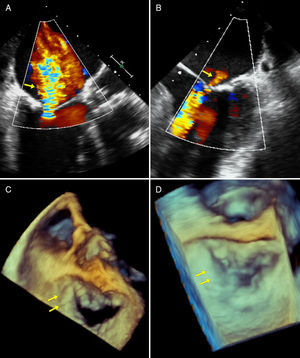
Aortic annulus diameter was 21 mm and right femoral artery diameter was 7.7 mm. The patient was scheduled for TAVR. During the implantation of a 23 mm Edwards SAPIEN X T valve, sudden severe LV dysfunction occurred with posterior-inferior akinesis and acute severe mitral regurgitation. Moderate central aortic prosthetic regurgitation was observed on aortography (appearing lighter on Doppler echocardiography) (Figures 1 and 2). Emergency coronary catheterization showed an acute occlusion of the mid right coronary artery (Figure 1). A thrombus was aspirated using a Pronto extraction catheter (Vascular Solutions, Minneapolis, MN, USA). Its shape suggested that it had been released from the pigtail catheter in the ascending aorta, even though heparin 100 U/kg had been administered and activated clotting time was 250 s (Figure 1). As soon as the right coronary artery was opened and TIMI 3 flow was restored, LV function recovered completely and the mitral regurgitation almost disappeared, returning to baseline status (Figures 2 and 3). Repeat aortography showed resolution of central prosthetic aortic valve regurgitation (Figures 1 and 2). The patient progressed without problems, with minimal troponin elevation and continuing normal LV function.
(A) Aortography with mild to moderate regurgitation (arrows) through the prosthetic aortic valve; (B) aortography showing resolution of regurgitation after removal of thrombus from the right coronary artery (CA) (due to immediate improvement of left ventricular function seen on transesophageal echocardiography); (C) acute thrombotic occlusion in the mid third of the RCA; (D) RCA with TIMI 3 flow after extraction of the thrombus; (E) thrombus extracted from the RCA after aspiration with the Pronto device.
(A) Deep transgastric view at 0°: mild central aortic regurgitation (arrows) during acute ischemia and acute severe LV dysfunction; (B) deep transgastric view at 0°: disappearance of aortic regurgitation after resolution of ischemia and immediate improvement of left ventricular function; (C) mid-esophageal view at 120°: mild central aortic regurgitation (arrows) during acute ischemia; (D) mid-esophageal view at 120°: scarcely any traces of aortic regurgitation after resolution of ischemia.
(A) Mid-esophageal view at 120°: severe mitral regurgitation during ischemia; (B) mid-esophageal view at 0°: mild mitral regurgitation after extraction of thrombus; (C) three-dimensional view of mitral valve from left atrium during acute ischemia with posterior leaflet coaptation defect; (D) three-dimensional view of mitral valve from left atrium after resolution of ischemia with recovery of posterior leaflet coaptation.
Periprosthetic aortic regurgitation or paravalvular leaks and their repercussions on prognosis are well known,1–4 but acute central aortic regurgitation during TAVR secondary to acute LV dysfunction is a lesser-known phenomenon that resolves after acute improvement of LV function. The importance is in being aware of the problem, and having in mind that transient ventricular dysfunction might be the cause of regurgitation; this is reversible and different from aortic regurgitation due to overdilatation, in which case the mechanism is inadequate leaflet coaptation. The mechanisms of prosthetic regurgitation in the situation described are not well understood, but are probably related to hypotension secondary to acute LV failure, which causes poor leaflet coaptation; the valves need adequate systolic pressure to open and a certain diastolic pressure to close correctly, or a minimum gradient between aortic diastolic pressure (which was low in this case) and LV diastolic pressure (high at the time of occlusion in this case). The patient's blood pressure was 50/30 mmHg. Moreover, acute ischemic mitral regurgitation is well known and is frequently associated with papillary muscle ischemia. Regarding coronary occlusion due to embolism, the thrombus could have originated from the catheter or a clot attached to the prosthesis.6 Cases in which embolism is caused by materials swept from the aorta are also described.7 In our case, the thrombus shape suggested that its origin was in the catheter. Aspiration opened the coronary immediately and there was no need to place a stent.
In conclusion, periprosthetic aortic regurgitation or paravalvular leak is a well-known condition after TAVR. However, central regurgitation after TAVR has received less attention, as a rare case usually associated with the guide remaining through the valves during the procedure, or to overdilatation aimed at preventing paravalvular leak. Our case shows that central regurgitation may also occur after (usually ischemic) acute LV dysfunction and that the situation, if not the original condition, is reversible. Additionally, this can be a guiding sign to rule out unsuspected acute dysfunction.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.