Peak oxygen consumption (pVO2) is a key parameter for assessing the prognosis of heart failure with reduced ejection fraction (HFrEF). However, it is less reliable when the cardiopulmonary exercise test (CPET) is not maximal.
ObjectiveTo compare the prognostic power of various exercise parameters in submaximal CPET.
MethodsAdult patients with HFrEF undergoing CPET in a tertiary center were prospectively assessed. Submaximal CPET was defined as a respiratory exchange ratio ≤1.10. Patients were followed for one year for the primary endpoint of cardiac death and urgent heart transplantation (HT). Various CPET parameters were analyzed as potential predictors of the combined endpoint and their prognostic power (area under the curve [AUC]) was compared using the Hanley-McNeil test.
ResultsCPET was performed in 442 HFrEF patients (mean age 56±12 years, 80% male), of whom 290 (66%) had a submaximal CPET. Seventeen patients (6%) reached the primary endpoint. The cardiorespiratory optimal point (COP) had the highest AUC value (0.989, p<0.001), and significantly higher prognostic power than other tested parameters, with pVO2 presenting an AUC of 0.753 (p=0.001). COP ≥36 had significantly lower survival free of HT during follow-up (p<0.001) and presented a sensitivity of 100% and a specificity of 89% for the primary endpoint.
ConclusionCOP had the highest prognostic power of all parameters analyzed in a submaximal CPET. This parameter can help stratify HFrEF patients who are physiologically unable to reach a maximal level of exercise.
O pico do consumo de oxigénio (pVO2) é um parâmetro fundamental na avaliação do prognóstico de doentes com insuficiência cardíaca com fração de ejeção reduzida (IC-FEr). Contudo, é menos fiável quando a Prova de Esforço Cardiorrespiratória (PECR) não é máxima.
ObjetivoComparar o poder prognóstico de diversas variáveis de exercício no contexto de PECR subáxima (subPECR).
MétodosAnálise prospetiva de doentes adultos com IC-FEr submetidos a PECR num centro terciário. SubPECR foi definida por um quociente respiratório ≤1,10. O Endpoint combinado foi morte de causa cardíaca/transplantação urgente até aos 12 meses de follow-up. Várias variáveis da PECR foram avaliadas como potenciais preditores do endpoint combinado (Área sob a Curva - AUC) e o seu poder prognóstico foi comparado através do teste Hanley&McNeil.
Resultados442 doentes com IC-FEr foram submetidos a PECR (56±12 anos, 80% homens), dos quais 290 (66%) fizeram uma SubPECR. Registaram-se 17 eventos (6% dos doentes). O Ponto Ótimo Cardiorrespiratório (POC) apresentou a melhor capacidade preditiva (AUC – 0,989, p<0,001) e o seu valor prognóstico foi significativamente superior ao das restantes variáveis, com o pVO2 a apresentar um valor de AUC de 0,753 (p=0,001). Doentes com POC≥36 apresentaram uma sobrevida livre de eventos significativamente inferior (p<0.001), tendo este cut-off revelado uma sensibilidade de 100% e uma especificidade de 89% para o endpoint.
ConclusãoPOC foi a variável com melhor poder prognóstico no contexto de subPECR, pode refinar a estratificação de risco de doentes com IC-FEr incapazes de fazer uma PECR máxima.
The cardiopulmonary exercise test (CPET) is a powerful predictor of mortality in heart failure with reduced ejection fraction (HFrEF) and is used to guide patient referral for heart transplantation (HT) and mechanical circulatory support (MCS).1–9
The 2016 International Society for Heart Lung Transplantation (ISHLT) listing criteria for heart transplantation defined peak volume of oxygen consumption (pVO2) as a major criterion for listing patients for heart transplantation, however, it requires a maximal CPET.10 Many HFrEF patients cannot attain maximal effort during CPET, due to various reasons that have been pointed out (cardiac dysfunction during exercise, ventilatory abnormalities due to respiratory muscle fatigue or concurrent respiratory disease, concurrent comorbidities such as peripheral arterial disease or muscular abnormalities, frailty, side effects of medications, psychological factors, choice of protocol), which reduces the prognostic value of pVO2. Peak respiratory exchange ratio (RER) is traditionally used to define a maximal CPET, however the optimal peak RER cut-off is still unknown, with various position papers and guidelines using different cut-offs.10–13 In the event of a submaximal CPET, the minute ventilation to carbon dioxide output (VE/VCO2) slope may be considered for risk stratification according to current guidelines, despite a low level of evidence (class IIb, level of evidence C).10 Several studies have reported other submaximal CPET variables with prognostic power, such as mean response time, the O2 uptake efficiency slope (OUES), VO2 at the anaerobic threshold (AT) and the cardiorespiratory optimal point (COP).14,15
The present study seeks to assess and compare the prognostic power of various exercise parameters in submaximal CPET for risk stratification in patients with HFrEF.
MethodsEthicsThe investigation conforms to the principles outlined in the Declaration of Helsinki. The institutional ethics committee approved the study protocol and all patients provided written informed consent.
Study sampleWe performed a single-center analysis of 442 consecutive HF patients referred to our institution between 2009 and 2018 with left ventricular ejection fraction (LVEF) ≤40% and in New York Heart Association (NYHA) class II or III who underwent CPET. All the patients were referred for assessment by the HF team and possible indication for HT or MCS.
Study protocolProspective follow-up included initial assessment of each patient within one month with clinical data including HF etiology (ischemic vs. non-ischemic), implanted cardiac devices, medication, comorbidities, NYHA class, laboratory, electrocardiographic and echocardiographic data, CPET data, and Heart Failure Survival Score.
Exclusion criteria were as follows: age <18 years; planned percutaneous coronary revascularization or cardiac surgery; exercise-limiting comorbidities (cerebrovascular disease, musculoskeletal impairment, or severe peripheral vascular disease); or previous HT. Patients with elective HT during the follow-up period (patients who had indication for HT and a heart became available in the first year of follow-up) were censored from the analysis at the time of HT.
Follow-up and endpointAll patients were followed for 12 months from the date of completion of the above-mentioned complementary exams.
The primary endpoint was a composite of cardiac death and urgent HT occurring during an unplanned hospitalization for worsening inotrope-dependent HF. Data were obtained from outpatient clinic visits and review of medical charts, and were complemented with a standardized telephone interview with all patients at 12 months of follow-up.
Cardiopulmonary exercise testingA maximal symptom-limited treadmill CPET was performed using the modified Bruce protocol on a GE Marquette Series 2000 treadmill. Gas analysis was preceded by calibration of the equipment. VE, VO2 and VCO2 were acquired breath-by-breath using a SensorMedics Vmax 229 gas analyzer. Heart rate (HR) was measured by continuous electrocardiogram, blood pressure (BP) was obtained manually with a sphygmomanometer, and oxygen saturation was monitored by pulse oximetry. Standard spirometry was not available in all patients.
Patients were encouraged to perform exercise until the RER was >1.10. A submaximal CPET was defined as one with a peak RER ≤1.10.
pVO2 was defined as the highest 30-s average achieved during exercise and was normalized for body mass. AT was determined by combining the standard methods (V-slope preferentially and ventilatory equivalents). The VE/VCO2 slope was calculated by least squares linear regression, using data acquired throughout the exercise. COP was measured as the minimum value of the ventilatory equivalent for oxygen (minimum VE/VO2). Partial pressure of end-tidal carbon dioxide (PetCO2) was reported before exercise, at AT and at peak exercise in mmHg units, and increase during exercise until AT was achieved (PetCO2DIF=PetCO2 at AT–PetCO2 at rest) was also calculated. Peak oxygen pulse (POP) was calculated by dividing derived pVO2 by maximum HR during exercise and was expressed in ml per beat. Circulatory power was calculated as the product of pVO2 and peak systolic blood pressure and ventilatory power was calculated by dividing peak systolic BP by the VE/VCO2 slope. HR reserve was calculated as the difference between maximum HR achieved with exercise and resting HR. HR recovery in the first minute after exercise was defined as the difference between maximum HR achieved with exercise and HR one minute into recovery.
Various composite CPET parameters were also automatically calculated.
Statistical analysisAll analyses compare patients with submaximal CPET and patients with maximal CPET. Data were analyzed using IBM SPSS for Windows, version 24.0 (IBM SPSS Inc, Chicago, IL).
Baseline characteristics were summarized as frequencies and percentages for categorical variables, as means and standard deviations for continuous variables when normality was verified, and as medians and interquartile range when normality was not verified by the Kolmogorov-Smirnov test. The Student's t test for independent samples or the Mann-Whitney test when normality was not verified were used for analysis of the variables.
The predictive power of various CPET parameters regarding the primary endpoint was analyzed for the highest area under the curve (AUC) in follow-up. Cut-off values for variables were determined from receiver operating characteristic (ROC) curves so that the sum of sensitivity and specificity was maximized. The Hanley-McNeil test was used to compare two correlated ROC curves.16
Survival curves were determined using the Kaplan-Meier method for subjects with submaximal CPET.
ResultsOverview of submaximal and maximal cardiopulmonary exercise test groupsA total of 442 patients were enrolled in the study. The mean age of the overall population was 56.2±12.5 years, 80% were male and mean LVEF was 28.6±6.9%. A submaximal CPET (peak RER ≤1.10) was recorded in 66% of cases (290 patients). The baseline characteristics of both groups are presented in Table 1.
Baseline characteristics of submaximal and maximal cardiopulmonary exercise test groups.
| Overall (n=442) | Submaximal CPET (n=290) | Maximal CPET (n=152) | p for difference between groups | |
|---|---|---|---|---|
| Clinical and demographic data | ||||
| Age, years | 56.2±12.5 | 57.2±12.2 | 53.8±12.9 | 0.007 |
| Male, % | 354 (80.1%) | 221 (76.2%) | 127 (83.6%) | 0.073 |
| BMI, kg/m2 | 27.2±4.3 | 27.6±4.5 | 26.5±3.4 | 0.012 |
| Ischemic etiology, % | 211 (47.7%) | 136 (47.2%) | 71 (46.7%) | 0.919 |
| ACEi/ARB/ARNI, % | 423 (95.7%) | 271 (95.4%) | 145 (98.0%) | 0.183 |
| BB, % | 388 (87.8%) | 254 (89.1%) | 126 (85.1%) | 0.230 |
| MRA, % | 340 (76.9%) | 224 (78.6%) | 110 (74.0%) | 0.279 |
| Diabetes, % | 98 (22.2%) | 72 (26.6%) | 24 (16.7%) | 0.023 |
| CKD, % | 140 (31.7%) | 93 (37.3%) | 46 (34.1%) | 0.524 |
| Baseline AF, % | 112 (25%) | 71 (24.7%) | 37 (24.3%) | 0.943 |
| Baseline ICD, % | 271 (61.3%) | 187 (64.5%) | 79 (52.0%) | 0.011 |
| Baseline CRT, % | 114 (25.8%) | 75 (25.9%) | 36 (23.7%) | 0.616 |
| HFSS | 8.5±1.0 | 8.49±1.02 | 8.58± 1.02 | 0.373 |
| Laboratory data | ||||
| Creatinine, mg/dl | 1.4±0.7 | 1.1±0.4 | 1.9±0.9 | 0.330 |
| vSodium, mEq/l | 137.9±3.1 | 138.1±3.1 | 137.6±3.2 | 0.064 |
| NT-proBNP, pg/ml | 2224.2±2764.0 | 2363.5±3082.8 | 2021.4±2184.0 | 0.319 |
| Echocardiographic data | ||||
| LVEDD, mm/m2 | 35.5±5.9 | 35.9±5.8 | 34.8±5.9 | 0.333 |
| LVEF, % | 28.6±6.9 | 29.1±6.5 | 27.4±7.6 | 0.021 |
| MR III-IV, % | 65 (14.7%) | 42 (16.4%) | 23 (18.6%) | 0.564 |
| RV dysfunction, % | 55 (12.4%) | 42 (31.6%) | 8 (20%) | 0.157 |
| CPET data | ||||
| Delta HR during exercise | 51 (0-130) | 47 (0-130) | 61 (12-130) | <0.001 |
| HHR1 | 19 (8-79) | 18 (8-73) | 21 (10-79) | 0.076 |
| CPET duration, min | 9.6±4.4 | 8.9±4.1 | 11.0±4.5 | <0.001 |
| Peak RER | 1.07±0.11 | 1.01±0.08 | 1.17±0.06 | <0.001 |
| pVO2, ml/kg/min | 17.9±6.1 | 17.0±5.5 | 19.9±6.7 | <0.001 |
| VE/VCO2 slope | 33.8±9.5 | 33.9±8.8 | 33.4±10.5 | 0.551 |
| OUES | 2.1±1.8 | 2.1±1.8 | 2.0±1.7 | 0.593 |
| pVO2, ml/kg/min at AT | 13.1±4.5 | 12.8±4.1 | 13.6±5.4 | 0.437 |
| O2 pulse, ml/kg/beat | 0.14±0.06 | 0.14±0.07 | 0.14±0.04 | 0.616 |
| Circulatory power, mmHg.ml/kg/min | 2786.9±1578.8 | 2646.4±1656.9 | 3095.2±1394.9 | 0.005 |
| Ventilatory power, mmHg | 4.8±1.7 | 4.7±1.6 | 4.9±1.8 | 0.100 |
| COP | 29.6±7.4 | 29.2±6.8 | 29.5±8.8 | 0.894 |
| PetCO2 at rest, mmHg | 33.4±4.7 | 33.1±4.7 | 33.9±4.8 | 0.121 |
| PetCO2 at AT, mmHg | 36.7±5.9 | 36.4±5.6 | 37.2±6.5 | 0.184 |
| PetCO2DIF, mmHg | 3.3±3.7 | 3.2±3.6 | 3.3±3.9 | 0.939 |
Values are mean ± standard deviation or median (interquartile range).
p values are calculated by the Student's t test for independent samples or the Mann-Whitney U test as appropriate.
ACEi: angiotensin-converting enzyme inhibitors; AF: atrial fibrillation; ARB: angiotensin receptor blockers; ARNI: angiotensin receptor-neprilysin inhibitors; AT: anaerobic threshold; BB: beta-blockers; BMI: body mass index; CKD: chronic kidney disease; COP: cardiorespiratory optimal point; CPET: cardiopulmonary exercise test; CRT: cardiac resynchronization therapy; HFSS: Heart Failure Survival Score; HRR1: heart rate recovery in the first minute after finishing CPET; ICD: implantable cardioverter-defibrillator; LVEF: left ventricular ejection fraction; LVEDD: left ventricular end-diastolic diameter; MRA: mineralocorticoid receptor antagonists; MR: mitral regurgitation; OUES: oxygen uptake efficiency slope; PetCO2: partial pressure of end-tidal carbon dioxide; PetCO2DIF: PetCO2 increase until AT is achieved; pVO2: peak oxygen uptake; RER: respiratory exchange ratio; RV: right ventricular.
At one year, the primary endpoint had occurred in 30 (6.8%) patients, with 18 patients experiencing cardiac death and 12 patient undergoing urgent HT (Table 2). No patients required urgent MCS. A similar proportion of patients met the primary endpoint in both groups (5.9% vs. 8.6%, p=0.285), which also applied to the individual components of the primary endpoint (cardiac mortality: 3.1% vs. 5.9%, p=0.155; urgent HT: 2.8% vs. 2.6%, p=0.872).
Adverse events at 12-month follow-up.
| Overall, n (%) | Submaximal CPET, n (%) | Maximal CPET, n (%) | p | |
|---|---|---|---|---|
| Combined primary endpoint | 30 (6.8%) | 17 (5.9%) | 13 (8.6%) | 0.285 |
| Total mortality | 20 (4.5%) | 9 (3.1%) | 11 (7.2%) | 0.047 |
| Cardiac mortality | 18 (4.1%) | 9 (3.1%) | 9 (5.9%) | 0.155 |
| Sudden cardiac death | 10 (2.3%) | 5 (1.7%) | 5 (3.3%) | 0.293 |
| Death from worsening HF | 8 (1.8%) | 4 (1.4%) | 4 (2.6%) | 0.446 |
| Urgent HT | 12 (2.7%) | 8 (2.8%) | 4 (2.6%) | 0.872 |
CPET: cardiopulmonary exercise test; HF: heart failure; HT: heart transplantation.
All CPET parameters were analyzed for the highest AUC in the follow-up period (Table 3). In the submaximal CPET group, COP had the highest AUC value (0.989), followed by PetCO2 at AT (AUC=0.835) and VE/VCO2 slope (AUC=0.815). PP presented the lowest predictive power in this group (AUC=0.694). The Hanley-McNeil test (Table 4) revealed that COP's predictive power was significantly higher than that of other variables (p<0.05 for all), including pVO2 (p=0.004) and VE/VCO2 slope (p=0.026). Despite the high AUC value of both PetCO2 at AT and VE/VCO2 slope in comparison to pVO2, no statistically significant difference was found when the Hanley-McNeil test was applied. COP retained a high predictive ability for the study's primary endpoint, irrespective of patient age (≤50 vs. >50 years) and gender, HF etiology (ischemic vs. non-ischemic), heart rhythm (sinus rhythm vs. atrial fibrillation) or the presence of chronic kidney disease (CKD).
Area under the receiver operating characteristic curve analysis for the primary endpoint and Hanley-McNeil p-values for comparison between groups.
| Submaximal CPET | Maximal CPET | p | |||
|---|---|---|---|---|---|
| AUC | 95% CI | AUC | 95% CI | ||
| pVO2, ml/kg/min | 0.753 | 0.670-0.837 | 0.749 | 0.607-0.890 | 0.970 |
| VE/VCO2 slope | 0.815 | 0.742-0.889 | 0.750 | 0.648-0.852 | 0.532 |
| COP | 0.989 | 0.969-0.999 | 0.632 | 0.478-0.785 | 0.017 |
| OUES | 0.767 | 0.604-0.930 | 0.853 | 0.746-0.959 | 0.417 |
| Circulatory power, mmHg.ml/kg/min | 0.773 | 0.681-0.865 | 0.783 | 0.678-0.889 | 0.922 |
| Ventilatory power, mmHg | 0.806 | 0.706-0.905 | 0.764 | 0.654-0.874 | 0.685 |
| Peak oxygen pulse, ml/kg/beat | 0.694 | 0.598-0.790 | 0.810 | 0.700-0.919 | 0.264 |
| PetCO2 at rest, mmHg | 0.712 | 0.588-0.836 | 0.573 | 0.410-0.736 | 0.223 |
| PetCO2 at AT, mmHg | 0.835 | 0.744-0.926 | 0.708 | 0.592-0.825 | 0.231 |
| PetCO2DIF, mmHg | 0.755 | 0.647-0.862 | 0.787 | 0.697-0.877 | 0.767 |
AT: anaerobic threshold; AUC: area under the curve; CI: confidence interval; COP: cardiorespiratory optimal point; OUES: oxygen uptake efficiency slope; PetCO2: partial pressure of end-tidal carbon dioxide; PetCO2DIF: PetCO2 increase until AT is achieved; pVO2: peak oxygen uptake; submaximal CPET: submaximal exercise test.
Hanley-McNeil p-values for area under the receiver operating characteristic curve comparison in the submaximal cardiopulmonary exercise test group.
| pVO2 | VE/VCO2 slope | COP | PetCO2 at AT | |
|---|---|---|---|---|
| pVO2 | 0.517 | 0.004 | 0.390 | |
| VE/VCO2 slope | 0.517 | 0.026 | 0.829 | |
| COP | 0.004 | 0.026 | 0.048 | |
| OUES | 0.894 | 0.641 | 0.013 | 0.507 |
| Circulatory power | 0.837 | 0.657 | 0.007 | 0.511 |
| Ventilatory power | 0.583 | 0.923 | 0.021 | 0.757 |
| Peak oyxgen pulse | 0.558 | 0.218 | <0.001 | 0.150 |
| PetCO2 at rest | 0.687 | 0.299 | 0.006 | 0.214 |
| PetCO2 at AT | 0.390 | 0.829 | 0.048 | |
| PetCO2DIF | 0.984 | 0.537 | 0.006 | 0.418 |
AT: anaerobic threshold; COP: cardiorespiratory optimal point; OUES: oxygen uptake efficiency slope; PetCO2: partial pressure of end-tidal carbon dioxide; PetCO2DIF: PetCO2 increase until AT is achieved; pVO2: peak oxygen uptake.
In the maximal CPET arm, COP presented a low predictive power: AUC=0.632, with a significant difference in its predictive power between the groups (p=0.017) (Table 3). OUES and PP were the variables with the highest AUC values, 0.853 and 0.810, respectively. No statistically significant difference between submaximal and maximal CPET was found regarding other variables, including pVO2 (p=0.970) and VE/VCO2 slope (p=0.532).
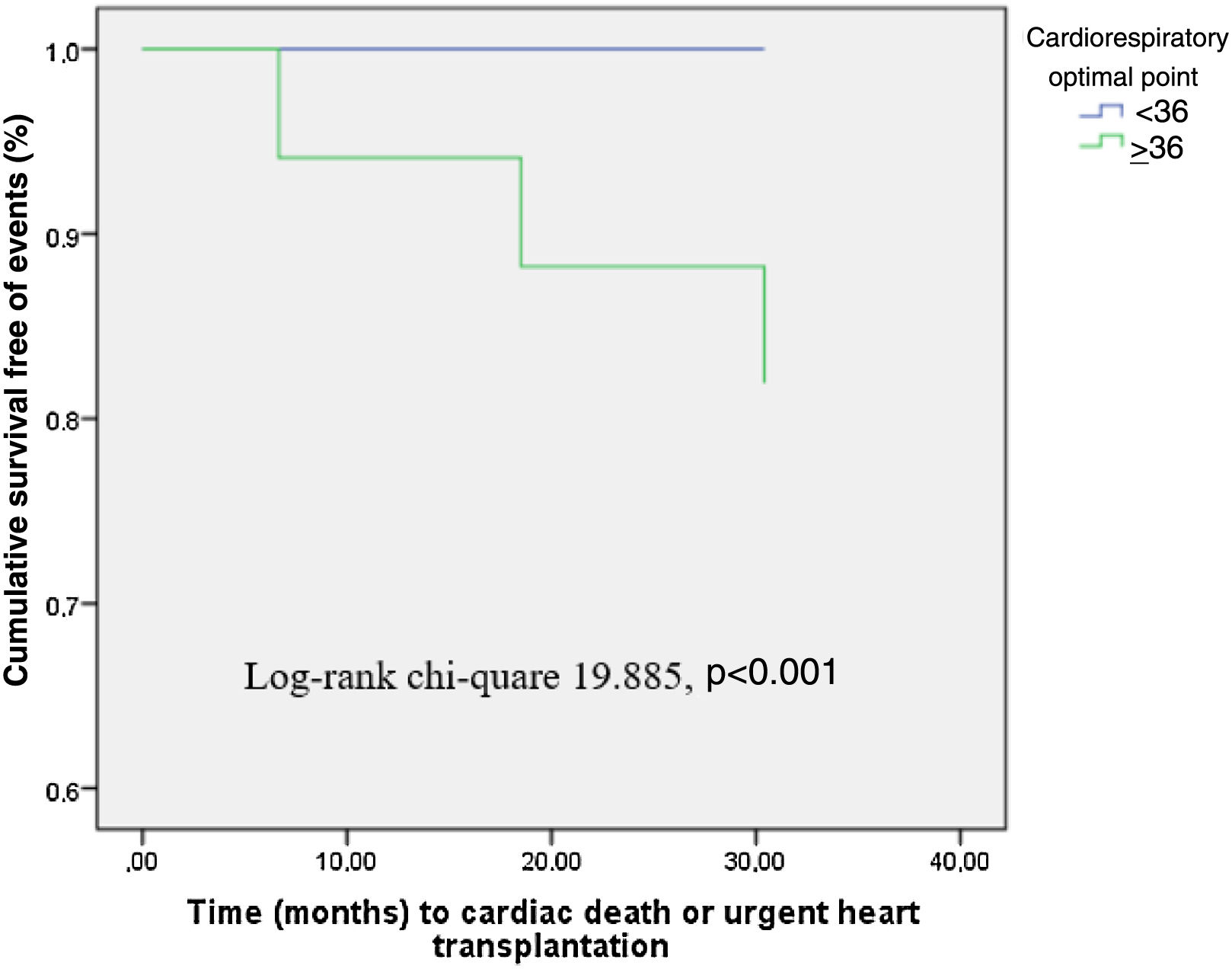
Cut-off values for variables were determined from the ROC curves so that the sum of sensitivity and specificity was maximized. A COP of 36 had a sensitivity of 100% and a specificity of 89% for the primary endpoint. Patients with peak RER ≤1.10 presenting a COP value of less than 36 had a significantly lower 12-month survival free of events (p<0.001) (Figure 1).
DiscussionIn HF patients, CPET is a powerful predictor of morbidity and mortality and has a central role in informing patient selection for advanced HF interventions, such as HT and MSC, with pVO2 and VE/VCO2 slope being the most used risk assessment tools.10 However, several other CPET variables have been shown to predict HF events, some of which can improve clinical stratification of HF patients when used together with the above-mentioned variables (exercise oscillatory ventilation, PetCO2 variation during exercise testing, HR recovery, PP, systolic blood pressure, and the ECG response to exercise).13
Although it is a well-established and powerful prognostic parameter, pVO2 is influenced by various factors such as patient effort, motivation, and familiarity with the procedure, body mass index, test protocol, non-cardiac effort limitation, and test termination criteria used, such that it loses some of its predictive ability in submaximal CPET, which is frequent in this population.14–16 In this regard, ISHLT established that for submaximal CPET, the VE/VCO2 slope may be considered for risk stratification according to current guidelines, despite a low level of evidence.10 Several other studies have investigated the role of other CPET variables in a submaximal setting, such as OUES and COP.
Overall, in our study there was no difference between groups (5.9% vs. 8.6%, p=0.285) regarding the primary endpoint. Nevertheless, the maximal CPET group presented higher 12-month total mortality, due to more non-cardiovascular death.
COP represents the lowest value of the oxygen ventilatory equivalent (the ratio between ventilation and oxygen consumption) in a given minute. It has proved to be a simple, practical, and reliable indicator that is free from observer error, occurring at modest exercise levels (30-50% of VO2). It is an established predictor of all-cause mortality in both healthy individuals and HF patients, especially when combined with pVO2,17,18 and has been shown to retain its value in a submaximal setting.19 In our study it presented the highest predictive power, with an AUC of 0.989, substantially higher than other tested parameters. A cut-off of 36 presented 100% sensitivity and 89% specificity for the primary endpoint. Interestingly COP retained high predictive power irrespective of patients’ gender, age (comparing patients younger and older than 50 years), HF etiology and baseline heart rhythm and the presence of CKD.
COP presented an AUC that was significantly higher than other variables in the submaximal CPET arm (p<0.005 for all study variables), showing that it is the most powerful predictor of our study's primary endpoint, in line with the findings of previous analysis. Its ability to predict events was higher than that of PetCO2 (p=0.048). However, it was the only tested parameter to display a significantly different performance between the tested groups (p=0.017), with a low predictive value in the maximal CPET group.
When the Hanley-McNeil test was applied to the other parameters, no statistically significant difference was found, despite numerical differences between AUC values.
Several investigations have documented that VE/VCO2 slope is a powerful exercise predictor of mortality in HF patients unable to perform a maximal effort,20–23 such that in the first 50% of exercise and below the respiratory compensation point it could provide suitable prognostic surrogates in these patients.24 In our study, it had the third highest AUC value (0.815), with a value lower than 36 achieving a sensitivity of 81.3% and a specificity of 71% for the primary endpoint.
PetCO2 is correlated with cardiac output in HF patients and can reflect disease severity. Resting, AT and peak exercise PetCO2, as well as its increase until AT, demonstrate independent prognostic value in this population. Guazzi et al. reported that a resting PetCO2 less than 33.0 mmHg and an increase during exercise of less than 3 mmHg were associated with a worse outcome.13,25 In our population, PetCO2 at AT presented the second highest AUC (0.835) in the submaximal CPET arm, with a cut-off of 33 mmHg revealing a sensitivity of 86.7% and a specificity of 73.0% for the primary endpoint. Neither resting PetCO2 nor its increase until AT had an AUC value numerically higher than pVO2.
OUES is a non-linear measure of ventilatory response to exercise and describes the relationship between VO2 and VE during incremental exercise. Its prognostic value has been reported to be superior to that of other CPET variables, without requiring a maximal effort by the patient.26–30 It had an AUC between those of pVO2 and VE/VCO2 slope (0.767), with values less than 1.5 having a sensitivity of 76.5% and a specificity of 80.5% for the combined endpoint.
Study limitationsThere are limitations to our study that should be noted. This was a single-center experience, and therefore the results may reflect local practice and results may not be applicable to other HF centers.
There is no consensus about the optimal peak RER cut-off to define maximal effort, especially in HF patients. The ISHLT guidelines10 use a peak RER of 1.05 as a cut-off but other documents propose different cut-offs, ranging between 1.0 and 1.10.10–13 In this study we chose a peak RER of 1.10 to define a maximal CPET.
Although there have been few studies assessing the performance of submaximal CPET parameters, our study's population has a similar clinical profile to that of other studies in this area (including age, prevalence of ischemic heart disease, diabetes and CKD and device implantation rates), with high rates of neurohormonal blockade drug therapy. However, HF may have been less severe in our population than in other studies, as LVEF and pVO2 were higher, and there was a low rate of events (particularly urgent HT), which may compromise the reproducibility of our results.31
Since subjects were referred for assessment by the HF team and possible indication for advanced therapies, they may not be representative of older HF patients or those with more comorbidities.
Finally, a long-term follow-up is crucial for effective validation of our results.
ConclusionsA substantial proportion of patients with HRrEF are unable to perform a maximal CPET, which compromises the predictive ability of several traditionally used CPET parameters. COP had the highest predictive power of all parameters analyzed in submaximal CPET, with a value ≥36 achieving a sensitivity of 100% and a specificity of 89% for the primary endpoint. The present study shows that this previously validated and easily-obtained parameter can provide prognostic information regarding HF events in patients unable to perform maximal exercise.
Author contributionsConception and design of the research: Reis JF, Gonçalves AV, Bras P, Soares R; data acquisition: Reis JF, Gonçalves AV, Bras P, Pereira-da-Silva T, Soares R; data and statistical analysis and writing of the manuscript: Reis JF; critical revision of the manuscript for intellectual content: Pereira-da-Silva T, Soares R, Timóteo AT, Ferreira RC.
Conflict of interestThe authors declare not to have any conflict of interest.