Intense and regular physical exercise is responsible for various cardiac changes (electrical, structural and functional) that represent physiological adaptation to exercise training. This remodeling, commonly referred to as ‘athlete's heart’, can overlap with several pathological entities, in which sudden cardiac death may be the first clinical presentation. Although pre-competitive screening can identify athletes with life-threatening cardiovascular abnormalities, there are no widely used standardized pre-participation programs and those currently implemented are controversial. Data from personal and family history, features of physical examination and changes in the 12-lead electrocardiogram can raise the suspicion of cardiac disease and lead to early detection of entities such as hypertrophic cardiomyopathy. However, interpreting the electrocardiogram is often challenging, because some changes are considered physiological in athletes. Thus, clinical decision-making in such cases can prove difficult: missing a condition associated with an increased risk of life-threatening events, or conversely, mislabeling an athlete with a disease that leads to unnecessary disqualification, are both situations to avoid. This paper provides an up-to-date review of the physiological cardiac effects of exercise training and highlights key points that should be taken into consideration in the assessment of young competitive athletes.
A prática intensa e regular de exercício físico é responsável por diversas alterações cardíacas, desde eléctricas, estruturais ou funcionais, que representam adaptações fisiológicas ao exercício. Esta remodelagem, frequentemente denominada por coração de atleta, pode mimetizar alterações típicas de diversas patologias, nas quais a morte súbita pode ser a primeira apresentação clínica. Apesar do rastreio pré-competição poder identificar atletas com alterações cardíacas potencialmente fatais, os programas de rastreio não estão estandardizados e aqueles já implementados permanecem controversos. Dados da história clínica pessoal e familiar, achados do exame físico e alterações no eletrocardiograma de 12 derivações, podem aumentar a suspeita de doença cardíaca e levar à deteção precoce de entidades como a miocardiopatia hipertrófica. Contudo, a interpretação do eletrocardiograma é frequentemente desafiante porque várias alterações são consideradas fisiológicas em atletas. Assim, as decisões clínicas são por vezes difíceis: não diagnóstico de condições associadas a um risco aumentado de eventos fatais, ou por outro lado, o diagnóstico errado de patologia cardíaca em atletas saudáveis pode originar a realização de exames de diagnóstico desnecessários ou a desqualificação inapropriada do atleta. Este artigo fornece uma revisão atualizada dos efeitos cardíacos fisiológicos do exercício físico e realça pontos-chave que deverão ser tidos em consideração na avaliação de atletas jovens de competição.
A sedentary lifestyle is associated with the development of cardiovascular (CV) risk factors, progression of coronary disease and occurrence of adverse clinical events.1,2 Health promotion efforts aimed at CV disease prevention, emphasizing physical activity, have led to increased participation in recreational and competitive sport.3 However, cases of sudden cardiac death (SCD) among athletes continue to raise concerns regarding the safety of exercise. A higher risk of coronary events and malignant arrhythmias during vigorous exercise has been described (the so-called exercise paradox), but the incidence of exercise-related cardiac arrest in individuals aged under 35 years has recently been shown to be low.4,5 Nonetheless, some doubts persist regarding the impact of prolonged and intense exercise training. In a study of 102 marathon runners, 12% had myocardial scarring on cardiac magnetic resonance imaging (MRI) with variable patterns (ischemic and non-ischemic), a finding three times more common than in age-matched controls.6 The role of exercise-induced fibrosis as arrhythmogenic substrate is as yet poorly understood. Some authors argue that a high volume of endurance exercise is responsible for adverse CV effects and that there may be a upper limit beyond which the adverse effects outweigh the benefits.7
Exercise training induces a constellation of physiological CV adaptations. The first descriptions of heart enlargement in athletes date to the 1890s, when increased cardiac size was demonstrated with chest auscultation and percussion in Nordic skiers and university runners.8,9 In the 1940s, a higher prevalence of resting sinus bradycardia was reported among Boston marathon runners.10 Nearly four decades later, in 1975, with the development of M-mode echocardiography, Morganroth described different left ventricular (LV) remodeling according to the type of exercise: concentric hypertrophy for strength and eccentric for endurance exercise,11 an observation that came to be known as the ‘Morganroth hypothesis’. However, with the increasing numbers of athletes being assessed, along with the development of cardiac imaging and the growth of published data in sports cardiology, it has been ascertained that other factors influence cardiac remodeling.
Early identification of athletes at higher risk of SCD is a cornerstone of screening. A wrong diagnosis could have serious adverse consequences: under-diagnosis of pathology may lead to life-threatening events being missed, and over-diagnosis may result in unnecessary disqualification. The ideal balance has not been struck, and the cost-effectiveness of current screening programs remains controversial. While a clinical history and physical examination are consensual, the same is not true for the electrocardiogram (ECG). Electrical and structural cardiac remodeling can induce ECG changes considered normal in athletes but pathological in non-athletes. Standardization of ECG interpretation in athletes could reduce the rate of false positives and the need for further investigations.
This paper provides an up-to-date review of exercise-induced physiological cardiac effects and an overview of the key points that should be highlighted in the assessment of athletes.
Molecular mechanisms and physiology of exerciseSeveral complex mechanisms have been postulated to account for the beneficial effects of physical activity. Continuous exercise training decreases myocardial oxygen demand, improves myocardial perfusion, promotes an antithrombotic environment, balances the autonomic system and prevents the development of CV risk factors such as hypertension, dyslipidemia, obesity and diabetes.3,12–15 At the molecular level, exercise enhances antioxidant capacity, induces myocardial heat shock proteins, and increases nitric oxide production and anti-apoptotic protection.15,16 Transient myocardial ischemia during regular bouts of exercise increases tolerance to subsequent ischemic stress, limiting ischemia-reperfusion injury and reducing the risk of lethal arrhythmias, a mechanism known as myocardial ischemic preconditioning.16,17
Physiological adaptations to exercise include a complex network of mechanisms (structural, neurohumoral, autonomic, metabolic and regulatory), which increase cardiac output.15 During exertion, increased skeletal muscle blood flow and oxygen extraction can raise cardiac output up to six times above baseline levels. The autonomic nervous system is involved in this process through parasympathetic withdrawal and sympathetic activation.17 Increased stroke volume results from increased ventricular end-diastolic volume and, to a lesser degree, from sympathetically-mediated reduction in end-systolic volume.18 By contrast, maximum heart rate does not significantly increase with regular exercise training.19 Post-exercise hemodynamics returns to baseline with vagal reactivation, a process that is enhanced in highly trained athletes.17 The effects of prolonged exercise on the right ventricle (RV) and left ventricle (LV) are different. During exercise there is a marked biventricular increase in cardiac output, but the decrease in vascular resistance is less pronounced in the pulmonary circulation. This mismatch between increased flow and vasodilation may result in an abnormal increase in pulmonary artery pressure and RV afterload, with a marked increase in RV workload. La Gerche et al.20 showed that RV volumes increase after endurance races, whereas LV volumes decrease, resulting in decreased RV but not LV ejection fraction (LVEF). Although short-term recovery appears to be complete, the long-term clinical significance of these changes is unknown.
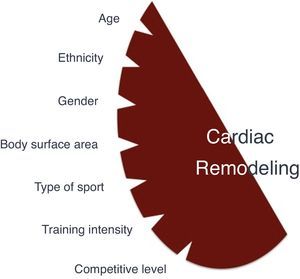
Factors influencing cardiac adaptations to exercise trainingSeveral factors, especially demographic and sport-related, are associated with more pronounced cardiac remodeling (Figure 1).
AgeAlthough most studies are on adult athletes (18–35 years old), cardiac adaptation is also evident in the younger population. A higher prevalence of ECG changes including LV hypertrophy, left atrial (LA) and right atrial (RA) enlargement, was shown in 1000 junior athletes compared with non-athletic controls.21 Some changes reflect normal variations at specific ages, such as the juvenile ECG pattern in individuals aged under 16. In a study of 1050 athletes (9–55 years old), those under 20 had significantly more ECG changes.22 Trained adolescents showed greater LV wall thickness (LVWT) than non-athletes.23 The range of LVWT differed between adolescent and adult athletes, with an upper limit of 16 mm in adults and 14 mm in adolescents, and LVWT >12 mm in ≈2% and 0.5% respectively.23,24 This suggests that LVWT limits should be lower in younger athletes. Less skeletal muscle mass, lower accumulated training, lack of testosterone before puberty and lower catecholamine responses to exercise in young athletes may help account for these differences. Many children are involved in intensely competitive activities early in life, but the effects of exercise training in very young athletes are unknown. Conversely, athletes are increasingly competing at older ages, and the impact of lifelong exercise training in veteran athletes is yet to be ascertained.
GenderCardiac remodeling is more pronounced among male athletes. Pelliccia et al. showed 23% lower LVWT and 11% smaller LV size in female compared to male athletes with similar age and training intensity.25 In this study no female had LVWT >12 mm. Sharma et al. showed similar findings in adolescent athletes (11% lower LVWT and 6% smaller LV size in females).25 Almost 5% of male athletes had LVWT ≥12 mm, but no females had LVWT >11 mm. Physiological ECG changes are also more frequent in male athletes.23 It should also be noted that a greater proportion of males are involved in extreme endurance and competitive sports, and hormonal factors may explain the gender-specific differences in these studies.
EthnicityBlack (Afro-Caribbean) athletes exhibit more pronounced cardiac remodeling than their white counterparts. Basavarajaiah et al. reported LVWT >12 mm in 18% black male athletes compared to 4% in white male athletes.26 Additionally, 3% of the black athletes, but none of the whites, exhibited LVWT ≥15 mm. The predominance of physiological remodeling in black athletes is not restricted to male gender. Rawlins et al. showed LVWT >12 mm in 3.3% black female athletes (all 12–13 mm), but none of their white counterparts had LVWT >11 mm.27 The prevalence of ECG abnormalities is also gender-dependent in athletes under 17 years of age.27–29 This ethnic discrepancy can be explained by differences in blood pressure modulation,30 endothelial function,31 arterial stiffness,32 angiotensin-converting enzyme gene I/D polymorphisms33 and insulin-like growth factor-1 expression.34
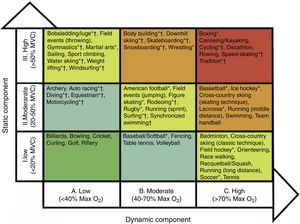
Sport-related factorsHemodynamic and cardiac adaptations vary according to the type and intensity of exercise. Isotonic exercise, also referred to as endurance or dynamic exercise, increases maximum oxygen consumption and cardiac output, with normal or reduced peripheral vascular resistance. In sports such as cross-country skiing, long-distance running, tennis or soccer, volume overload is predominant. In contrast, isometric exercise, also known as strength or static exercises, substantially increases blood pressure and peripheral vascular resistance, with normal or only slightly elevated cardiac output. Sports such as climbing, gymnastics, wrestling and body-building predominantly produce pressure overload. Endurance sports generally induce more pronounced cardiac remodeling. However, sports cannot be classified simply on the basis of this dichotomy. Many disciplines combine elements of both endurance and strength exercise, and it can be difficult to identify the predominant component. Mitchell et al. proposed a classification of sports according to the intensity of exercise in static and dynamic components35 (Figure 2). Although widely used, this classification has some limitations. Factors such as emotional stress during competition and environmental conditions were not considered. Emotional stress activates the sympathetic drive, leading to increased catecholamine concentrations, heart rate, blood pressure and myocardial contractility. These responses increase myocardial oxygen demand, potentially triggering arrhythmias and myocardial ischemia. Thus, during competition, sports associated with low myocardial oxygen demand relative to the exercise required (e.g. golf or billiards) can be associated with higher emotional stress. Environmental factors such as altitude, temperature, humidity and air pollution are responsible for different myocardial workloads, and should also be considered. Some sports have specific risks that should be borne in mind, such as those involving physical contact or projectiles (risk of commotio cordis) or those involving high velocity (risk of vertebral artery dissection due to abrupt head hyperextension).
Sports classification based on peak static and dynamic components achieved during competition. The lowest total cardiovascular demands (cardiac output and blood pressure) are shown in green and the highest in red. Max O2: maximal oxygen uptake; MVC: maximal voluntary contraction. *Danger of bodily collision. †Increased risk if syncope occurs (adapted from Mitchell et al. in the report of the 36th Bethesda Conference35).
Since the first reports by Morganroth et al.,11 several studies have shown a direct relationship between exercise training and cardiac structural remodeling (Table 1). Compared to non-athletes, athletes have 15–20% greater LVWT and 10–15% greater LV size. In a landmark study of 1309 Italian athletes engaged in 38 sports, 45% had LV end-diastolic diameter ≥55 mm (≥60 mm in 14%).36 A markedly dilated LV was more common in athletes with a larger body surface area and in those participating in endurance sports (cycling, cross-country skiing, and rowing/canoeing). Indexed LV mass exceeded the upper limits in 9% of male and 7% of female athletes. Another study of 286 professional cyclists revealed a dilated LV in 35%, even after adjustment for body surface area.37 Regarding LVWT, Italian studies showed LVWT >12 mm in a small percentage of elite athletes (1.1%–1.7%),23,36 with concomitant LV dilation. It should be emphasized that LVWT >13 mm and LV dimensions >65 mm are rare in healthy athletes. Analysis of these studies raises some concerns: they were cross-sectional in design and included mainly white male athletes; they were performed during competitive seasons, used M-mode or two-dimensional echocardiography, and controls were age- and gender-matched sedentary individuals.
Data of studies reporting left ventricular dimensions in athletes.
| Study | n | Sport | Age (y) | Male (%) | Ethnicity | LVEDD | LVWT |
|---|---|---|---|---|---|---|---|
| Pelliccia et al.23 | 947 | Multi (25) | 22±3 | 78 | – | ≥55 mm: 38% ≥60 mm: 4% Male: 54.2±4.0 mm (40–66) Female: 48.4±3.7 mm (40–61) | >12 mm: 1.7% Male: 10.1±1.2 mm (7–16) Female: 8.4±0.9 mm (6–11) |
| Pelliccia et al.36 | 1309 | Multi (39) | 24±6 | 73 | – | ≥55 mm: 45% ≥60 mm: 14% Male: 55.5±4.3 mm (43–70) Female: 48.4±4.2 mm (38–66) | >12 mm: 1.1% Mean: 9.3±1.4 mm (5–15) |
| Sharma et al.23 | 720 | Multi (13) | 16±1 | 75 | White (98%) | Male: 51.6±3.3 mm (42–60) Female: 47.7±3.3 mm (41–55) | ≥12 mm: 4% Mean: 9.5±1.7 mm (6–14) |
| Abergel et al.37 | 286 | Cycling | 28±3 | 100 | White | >60 mm: 51% Mean: 60.1±3.9 mm (49–73) | >13 mm: 8.7% Mean: 11.1±1.3 mm (7–15) |
| Di Paolo et al.29 | 154 | Soccer | 16±1 | 100 | Black | >54 mm: 16% >60 mm: 0.6% Mean: 51.0±3.6 mm (42–62) | >12 mm: 2.6% Mean: 10±1 mm (6–13) |
| Basavarajaiah et al.26 | 300 | Multi (6) | 21±6 | 100 | Black | Mean: 53±4.4 mm (44–64) | >12 mm 18% Mean: 11.3±1.6 mm (8–16) |
LVEDD: left ventricular end-diastolic diameter; LVWT: left ventricular wall thickness; Multi: multiple; y: years.
Although broadly accepted, the validity of the Morganroth hypothesis is questioned by some authors.38 Spence et al. prospectively analyzed untrained subjects randomized to supervised endurance (n=10) or resistance (n=13) exercise, with assessments at baseline, after six months of training and six weeks after detraining.39 LV adaptation, including increased LV mass and LVWT, were only present in endurance athletes. Despite the small sample and relatively short training period, these results cast some doubt on the importance of remodeling in response to resistance exercise training.
LA dilation is also a physiological adaptation in trained athletes. Pelliccia et al. reported a 20% increase in LA dimensions (M-mode transverse dimension ≥40 mm) among 1777 athletes engaged in 38 disciplines,40 while D’Andrea et al. showed a 28% increase in indexed LA volume in 615 endurance athletes.41 The increased LA size can be explained by concomitant LV cavity enlargement and volume overload. Although not completely understood, LA remodeling may be one of the mechanisms associated with supraventricular arrhythmias in athletes.
Due to its unusual shape, which makes it more difficult to image, the effects of exercise on RV structure have been neglected. The RV also enlarges in response to continuous exercise training, supporting the concept of balanced biventricular enlargement.42 Zaidi et al. confirmed greater RV dimensions in athletes compared to non-athletes, with smaller dimensions in black athletes.43 MRI studies have confirmed RV enlargement, mainly in endurance athletes.44
The association of exercise and aortic root dilation is inconsistent. The largest study published (n=2317) showed that dilated aortic root is uncommon among competitive athletes: ≥40 mm in 1.3% of males and ≥34 mm in 0.9% of females (99th percentiles).45 Dilatation of the aortic root is unlikely to represent a physiological adaptation, and is most likely an expression of pathology. The observation of a 2.5-fold increase in the aortic root over eight years of follow-up in male athletes with enlarged aortic root at initial assessment supports this fact. Other small studies report greater diameters in strength- compared to endurance-trained athletes, but also with a low prevalence of dilated aortic root.46,47
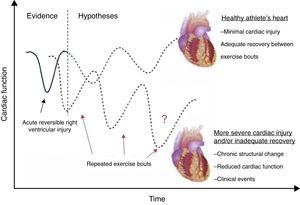
Functional adaptationFunctional physiological adaptations to exercise training are incompletely understood. LVEF is generally normal among athletes. Even extreme and uninterrupted endurance training over long periods of time appears not to be associated with deterioration in LVEF.48 A meta-analysis of 59 studies showed no difference in systolic and diastolic LV function among trained athletes compared with sedentary controls.49 However, a transient reduction in LVEF has been demonstrated after prolonged strenuous exercise, termed ‘cardiac fatigue’.50 The interpretation of these changes as physiological is not consensual. Some authors have questioned the real effects of competitive exercise on LV dynamics, particularly after prolonged endurance training, as reported in cyclists.37 The reduction of LVEF in endurance athletes is secondary to LV dilation, but performance-enhancing drugs could be involved. Nevertheless it should be stressed that indices of systolic function are of limited value in the assessment of ventricular performance at rest (due to the load dependence of LVEF and the fact that Doppler indices are only recorded in the systolic phase). New advances in echocardiography, including strain imaging and speckle tracking, suggest that intense exercise may lead to changes in LV systolic function that are not detected by LVEF assessment. These tools are more sensitive in assessing systolic adaptations to exercise, and hence allow early detection of systolic impairment.42,44,51 Baggish et al. found unchanged LVEF in 20 rowers after 90 days of training, but significant changes in all the direct measures of LV systolic function73: peak systolic tissue velocities increased; radial strain increased similarly in all segments; longitudinal strain increased with a base-to-apex gradient; and circumferential strain increased in the LV free wall but decreased in regions adjacent to the RV. Reductions in septal circumferential strain were strongly correlated with changes in RV structure and function. This finding may reflect a novel form of ventricular interdependence. In 16 ultramarathon athletes, peak RV strain decreased in the post-race period and a slight delay in time to peak strain relative to pulmonary valve closure was identified, while in the LV, peak circumferential, radial and longitudinal strain, and torsion decreased significantly after exercise.52 Reductions in these indices could be a consequence of intrinsic LV impairment or increased RV afterload. In fact, the RV is not a passive chamber, as previously thought, but plays a crucial role in cardiac adaptations to exercise. Intense endurance exercise causes acute RV dysfunction that recovers in the short term, but chronic structural changes and reduced RV function are evident in some athletes.19 This hemodynamic imbalance may promote transient RV injury or incomplete recovery, with possible long-term structural consequences (Figure 3).
LV diastolic function can be enhanced by prolonged exercise training.53,54 This improvement is essential to preserve stroke volume, mainly due to the ability of the LV to relax at high heart rates.55 Sustained endurance training preserves ventricular compliance with age, potentially preventing heart failure in the elderly.56 In strength sports LV relaxation appears to remain unchanged or mildly impaired, although concentric hypertrophy does occur.44
Electrical adaptationMore than 80% of competitive athletes have changes in resting ECG reflecting physiological adaptation to exercise training; changes potentially confounded with CV pathology are present in 10–14%.28,57,58 Guidelines and consensus documents on ECG interpretation in athletes have been published in recent years. The European Society of Cardiology (ESC) guidelines published in 201059 divide the ECG findings into two groups:
- •
Group 1: common and training-related changes;
- •
Group 2: uncommon and trained-unrelated changes.
Group 1 (normal findings) includes sinus bradycardia, first-degree atrioventricular (AV) block, incomplete right bundle branch block and isolated QRS voltage criteria for LV hypertrophy. The American guidelines, published in 2011, were in line with the ESC document,60 while in 2013, experts in sports cardiology and sports medicine defined standards to distinguish normal from abnormal ECG findings in athletes, known as the Seattle criteria (Table 2).61–63
The Seattle criteria for normal ECG findings in athletes (adapted from 61).
| 1. Sinus bradycardia (≥30 bpm) |
| 2. Sinus arrhythmia |
| 3. Ectopic atrial rhythm |
| 4. Junctional escape rhythm |
| 5. First-degree AV block (PR interval >200 ms) |
| 6. Mobitz type I (Wenckebach) AV block |
| 7. Incomplete RBBB |
| 8. Isolated QRS voltage criteria for LVH |
| • Except: QRS voltage criteria for LHV occurring with any non-voltage criteria for LVH such as LA enlargement, left axis deviation, ST segment depression, T-wave inversion or pathological Q waves |
| 9. Early repolarization (ST elevation, J-point elevation, J-waves, or terminal QRS slurring) |
| 10. Convex (‘domed’) ST segment elevation combined with T-wave inversion in leads V1–V4 in black athletes |
AV: atrioventricular; bpm: beats per minute; LA: left atrium; LVH: left ventricular hypertrophy: RBBB: right bundle branch block.
Electrical adaptations in athletes result from conditioning of the cardiac autonomic nervous system (increased vagal tone and/or sympathetic withdrawal) and structural remodeling. Increased vagal tone is responsible for findings such as bradycardia, sinus arrhythmia, early repolarization and first-degree Mobitz type I AV block, alterations that disappear with increased heart rate during exercise. Structural remodeling is responsible for criteria of increased cavity sizes.61,64
Electrical adaptations include the following:
- •
Resting bradycardia: present in 60%–80% of highly trained athletes, especially those engaged in endurance sports.21,28,29,65 In the absence of symptoms, a heart rate ≥30 bpm and/or pauses of ≥3 s during sleep hours should be considered normal.
- •
Sinus arrhythmia: an exaggerated response is found in >50% of athletes, resulting from variation during the respiratory cycle.21,65
- •
Ectopic atrial rhythm: P waves with different morphologies compared to the sinus P waves (known as a ‘wandering atrial pacemaker’ if there are more than two different morphologies) are normal in athletes and are most easily seen when P waves are negative in the inferior leads.
- •
Junctional escape rhythm: resulting from a faster QRS rate than the resting P wave rate; can be found in athletes with marked bradycardia.65
- •
First-degree AV block and Mobitz type I AV block: these are considered normal findings in athletes. The former is common (3%–14%) but the second is rare (<1%).21,28,29,64,65
- •
Incomplete right bundle branch block: present in 30%–40% of athletes, particularly in endurance disciplines,21,29,65 reflecting prolonged conduction time resulting from increased RV size secondary to regular training.24,29,65,66
- •
Isolated QRS voltage criteria for LV hypertrophy: found in approximately 45% of athletes. In black individuals >80% may be affected.21,65,66 Association with other markers of non-voltage criteria for LV hypertrophy (LA enlargement, left axis deviation, ST-segment depression, T-wave inversions or pathological Q waves) should prompt exclusion of pathological hypertrophy.60,61
- •
Early repolarization: present in >50% of highly trained athletes, reaching 80–90% in black athletes.29,65–68 Besides the strong association with ethnicity, early repolarization is more common in young male athletes and in those with increased QRS voltage, interventricular septal thickness and slower heart rate.67 Tikkanen et al. showed a significant association between early repolarization in the inferior leads and increased risk of SCD in middle-aged non-athletic subjects.68 This pattern is also common among athletes, and probably represents a dynamic process related to exercise intensity.67 To date, there is no evidence to support a relationship between early repolarization and SCD or other cardiac events in athletes. In a recent retrospective analysis of 118 male soccer players with early repolarization, no cardiac deaths occurred during a mean follow-up of 13.3 years.69 All the patterns of early repolarization – ST elevation at QRS end (J-point), J-wave with or without ST elevation, terminal QRS slurring with or without J-wave/new J-point – should be considered normal variants in athletes.61
- •
Repolarization in black athletes: more than two-thirds of black athletes exhibit ST-segment elevation and up to 25% show T-wave inversions.29,66 Convex (‘domed’) ST-segment elevation combined with T-wave inversion in leads V1–V4 is also frequent and in the absence of symptoms, positive family history or abnormal physical examination, does not require further assessment (Figure 4).
- •
Juvenile pattern: T-wave inversions in the right precordial leads are a normal ECG finding in athletes aged under 16, because of RV dominance and repolarization polarity directed posteriorly.65
In the interpretation of athletes’ ECG, it should be stressed that some normal cut-offs differ between the sexes (e.g. prolonged QT interval according to the Seattle criteria: female >470 ms; male >480 ms).70
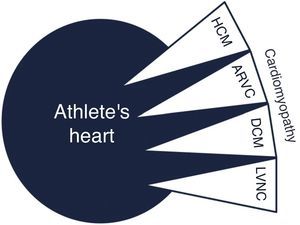
Overlap with cardiomyopathyIt can be challenging to distinguish exercise-related cardiac adaptations from cardiomyopathies (Figure 5).
The majority of dilemmas during the assessment of athletes occur when cardiac remodeling mimics phenotypical findings of hypertrophic cardiomyopathy (HCM). Almost 2% of adult male athletes show increased LVWT of 13–15 mm, falling into the gray zone between extreme expression of athlete's heart and a mild HCM phenotype.23 In female and adolescent athletes LVWT >12 mm is extremely rare and should be investigated further.22 As the phenotypic expression of HCM is incomplete during periods of rapid growth, younger athletes (prepubertal or pubertal) with suspected HCM should be closely monitored. The main differences between HCM and athlete's heart are depicted in Table 3. Compared to HCM patients, the hypertrophic pattern in athletes is uniform and associated with concomitant LV dilation. Reduction in LVWT (2–5 mm) after a period of detraining suggests athlete's heart, but compliance with detraining is difficult and requires the cooperation of athletes. It should be noted that in athletes with mild forms of HCM, increased LVWT could be related to both exercise and HCM and may decrease after detraining. Absence of response to detraining is suggestive of pathological LV hypertrophy, but regression with detraining does not necessarily represent only physiological hypertrophy. In contrast to physiological remodeling, in which diastolic function is preserved or even enhanced, HCM is characterized by abnormal LV relaxation indices and increased filling pressure; however, a normal diastolic filling pattern does not exclude pathological hypertrophy.
Differences between hypertrophic cardiomyopathy and athlete's heart.
| Investigation | Favors athlete's heart | Favors HCM |
|---|---|---|
| Family history | Absent | Known HCM diagnosis, SCD |
| Symptoms | Asymptomatic and good exertion tolerance | Pre-syncope, syncope, palpitations, breathlessness, chest pain, fatigue out of proportions to the degree of exertion |
| Physical examination | Unremarkable | Loud systolic murmur that increases with Valsalva maneuver, forceful apical impulse |
| ECG | Normal physiological adaptations (including isolated LVH criteria)a | High QRS voltage plus unusual findings Pathological Q waves ST-segment depression T-wave inversions in anterolateral leads LBBB |
| Echocardiogram | Symmetrical increased LVWT (usually <12 mm in female and junior athletes; rarely >13 mm in white male athletes) Normal or improved LV diastolic function Increased LV dimensions (LVEDD >55 mm) Balanced RV dilation | Asymmetric increased LVWT (>15 mm) LV diastolic dysfunction (impaired relaxation, increased deceleration time and filling pressures) Disproportionate LA dilatation Reduced LV dimension (LVEDD <45 mm) LVOT obstruction |
| CPET | VO2 max >45 ml/kg/min VO2 max >120% of predicted | VO2 max lower than predicted |
| MRIb | Absence of late enhancement or non-specific pattern (ischemic or non-ischemic, mainly in veteran endurance athletes) | Typical late enhancement (junction of RV and interventricular septum and in the thicker segments) |
| Detraining | Reduction in LVWT (2–5 mm)c | Persistence of increased LVWT |
| Genetic test | Negatived | Positive for mutation related to HCM |
CPET: cardiopulmonary exercise testing; ECG: electrocardiogram; HCM; hypertrophic cardiomyopathy; LBBB: left branch bundle block; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVH: left ventricular hypertrophy; LVOT: left ventricular outflow tract; LVWT: left ventricular wall thickness; MRI: magnetic resonance imaging; RV: right ventricle; SCD: sudden cardiac death.
In cases with some characteristics in the gray zone additional investigation is necessary. In cardiopulmonary exercise testing, while athletes achieve peak VO2 that typically exceed predicted values (usually >120%), in HCM patients only 1.5% exceed these values. MRI is useful for morphological characterization, particularly of apical LV segments, but also due to the additional information it provides, such as identification of myocardial fibrosis in HCM located in thicker myocardial segments and in RV insertion points of the interventricular septum. Although there is a significant rate of false negative results, genetic tests can help in difficult cases. A positive result for one of the known mutations of HCM establishes the diagnosis. Even without structural changes suggestive of HCM, athletes exhibiting abnormal and extensive ECG repolarization changes should be followed, because these changes can precede morphological features.
Dilated cardiomyopathyMarked LV dilatation may require a differential diagnosis between physiological adaptation and dilated cardiomyopathy (DCM), particularly when LV ejection fraction is in the lower normal range or depressed. As previously reported, 14% of athletes have LV diastolic diameter ≥60 mm, and it can reach 70 mm in men.36 In extreme endurance sports such as cycling, the prevalence and degree of LV dilation is greater, with a considerable proportion of athletes having concomitant decreased LVEF at rest, fulfilling criteria for a diagnosis of DCM.37 In these cases of extreme LV dilation, correct assessment of LVEF and exclusion of wall motion abnormalities are fundamental. DCM patients have reduced exercise capacity, reflected in low maximum VO2 and high VE/VCO2 slopes on cardiopulmonary exercise testing. MRI permits accurate assessment of LVEF and detection of fibrosis, usually mid-wall.
Arrhythmogenic right ventricular cardiomyopathyWith regard to arrhythmogenic right ventricular cardiomyopathy (ARVC), a relationship has been postulated between ultra-endurance exercise and an ARVC-like phenotype. Recently 3% black and 0.3% white athletes were found to meet criteria for ARVC, but further investigation did not detect any pathological findings.43 Extreme exercise can cause consecutive bouts of RV dilatation and dysfunction, which may alter the RV interstitial matrix. In favor of this hypothesis is the lower than expected prevalence of desmosomal gene mutations identified in endurance athletes with complex ventricular arrhythmias of RV origin.71 This supports the concept of ARVC being acquired through intense exercise. When ARVC is suspected the combination of findings from personal and family history, ECG, Holter ECG, transthoracic echocardiogram and MRI may establish the diagnosis.
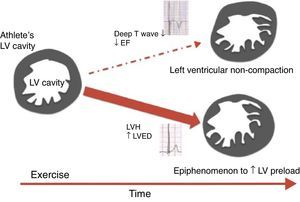
Left ventricular noncompactionLeft ventricular noncompaction (LVNC) is an unclassified cardiomyopathy for which the optimum diagnostic criteria are the subject of debate. The current criteria, based on echocardiography and MRI, reveal low specificity, leading to overdiagnosis of this entity. Furthermore, the LVNC phenotype can overlap with other cardiomyopathies such as HCM and DCM. Gati et al.58 showed a higher prevalence of LV trabeculation in athletes compared to controls (18.3% vs. 7.0%), with 8.1% fulfilling criteria for LVNC. Only a small proportion (0.9%) revealed reduced LVEF and marked repolarization changes in association with criteria for LVNC, raising the possibility of an underlying cardiomyopathy. LV trabeculations without other features of noncompaction or repolarization changes could be a physiological response to exercise in highly trained athletes (Figure 6). Changes in preload imposed by exercise could be the cause of these morphological changes, since LV trabeculations have been found in patients with heart failure, thalassemia and in pregnant women, conditions which are also associated with increased cardiac preload.
Scheme proposed for the significance of left ventricular trabeculation in athletes (adapted from 58). EF: ejection fraction; LV: left ventricular; LVED: left ventricular end-diastolic diameter; LVH: left ventricular hypertrophy.
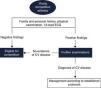
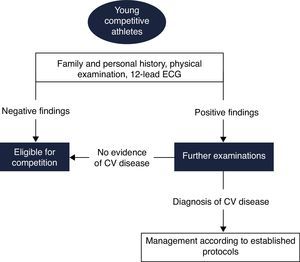
Pre-participation screening aims to detect silent CV abnormalities associated with risk of SCD. This issue has been the subject of intense debate, for which there is still no definite consensus. Few countries have implemented standardized screening protocols, and even among these, there is considerable heterogeneity. In 2005 the ESC published a consensus statement for CV pre-participation screening of young competitive athletes.72 This document was mainly based on the Italian experience, and advocates the inclusion of ECG in the screening protocol for athletes (Figure 7).
Proposed European Society of Cardiology screening protocol for young competitive athletes (adapted from 72). CV: cardiovascular; ECG: electrocardiogram.
Most of the conditions associated with a higher risk of SCD in athletes are genetically determined with an autosomal dominant inheritance pattern, and so the family history is of great importance. Family history should be considered positive in the presence of SCD, coronary events, or diagnosis of cardiomyopathies or primary inherited arrhythmias in first-degree relatives (male <55 years; female <65 years). Regarding personal history, care should be taken in the presence of CV symptoms, particularly if exercise-related. Chest pain, pre-syncope, syncope, irregular beats, palpitations, breathlessness or fatigue disproportionate to exertion are the most commonly reported symptoms. Syncope is relatively common among highly trained athletes (up to 6%), most frequently of benign origin due to reflex mechanisms, and sometimes occurring in the postexertional period. Although some cases suggest that syncope could be a manifestation of exaggerated vasodilatation, episodes during exertion may have a cardiac origin and should be comprehensively investigated, initially with transthoracic echocardiogram, Holter ECG and exercise testing, which are mandatory in the diagnostic workup of these athletes.73 Athletes with syncope or chest pain but normal echocardiogram and exercise testing should undergo coronary computed tomography angiography (CCTA) to exclude coronary artery anomalies. It is important to stress that coronary artery disease is the most frequent cause of SCD in athletes aged over 35, and it is essential to screen for CV risk factors in this population.
Physical examinationBeyond the conventional cardiac assessment, physical examination of athletes should focus on specific factors according to the suspected diagnosis (e.g. musculoskeletal features suggestive of systemic manifestations as phenotypic red flags for cardiomyopathies or Marfan syndrome). Athletes with positive findings should be referred for additional investigation, most commonly transthoracic echocardiogram, Holter ECG and exercise testing. Cardiac MRI, CCTA or invasive exams such as electrophysiological study may be required in specific situations. In the presence of extracardiac manifestations, collaboration of other specialists is important, such as neurologists in the case of abnormal neurological findings.
ElectrocardiogramThe role of the ECG in athletes’ screening is controversial, with arguments both for and against. Potentially lethal conditions with ECG manifestations account for approximately two-thirds of SCD in young competitive athletes – HCM, ARVC, DCM, long QT syndrome, Brugada syndrome, short QT syndrome and pre-excitation syndromes. Some ECG changes found in cardiomyopathies precede the morphological phenotype: the condition goes through molecular, electrical and morphological stages, with risk of life-threatening arrhythmias at all these stages. However, the ECG is unable to identify other causes of SCD such as premature coronary artery disease, congenital coronary anomalies or aortic root disorders.
Evidence supporting the use of ECG in screening of athletes came from the Veneto region of north Italy, where after long-term systematic screening the rate of SCD per 100 000 person-years declined from 3.6 to 0.4 in athletes but remained unchanged in unscreened non-athletes.74 However, some authors criticize these results, arguing that the study was not randomized, and countering with a study performed in Israel in which the mean annual SCD rate paradoxically increased after ECG screening (from 2.54 to 2.66/100 000).75 These results also should be interpreted with caution, because the SCD rate was based on media reports and the number of athletes was estimated.
In the USA pre-participation screening is performed using personal and family history and physical examination, but without ECG. This methodology is based on the assumption that the ECG in not cost-effective for screening of large populations of athletes due to the high false positive rate. However, screening without ECG appears to have a limited ability to detect potentially fatal abnormalities. In a retrospective analysis of 115 young athletes in the USA suffering SCD and previously screened, only 3% were suspected of having CV disease based on clinical history and physical examination and <1% were correctly diagnosed.76
Erroneous interpretation of the ECG may trigger expensive diagnostic tests and lead to unnecessary disqualification from competitive sports. This is more important for professional athletes in whom disqualification from competition has significant financial and psychological consequences. Conversely, signs of potentially lethal CV disorders may be misinterpreted as normal variants. It is essential to recognize that false positive ECG rates are strongly influenced by the criteria used, and so it is critical to improve physicians’ expertise in distinguishing normal adaptations from abnormal changes in athletes. Standardization with the Seattle criteria has led to improvement in the accuracy of ECG interpretation among several different physician specialties.77 Nevertheless, controversy regarding which ECG changes should be analyzed in athletes continues. Gati et al.78 recently showed that isolated axis deviation and atrial enlargement comprise a high burden of the ECG findings, but do not predict the presence of underlying structural or functional abnormalities. In this analysis, exclusion of these characteristics reduced the false positive rate from 13% to 7.5%.
Despite the intense debate concerning its efficacy and cost-effectiveness, the ECG is recommended by several organizations, including the International Olympic Committee, and is invariably performed in real-world clinical practice. Physicians responsible for assessment of athletes should therefore be able to identify exercise-induced ECG adaptations, and in the case of abnormal findings to set in motion appropriate investigation and management. Table 4 shows some arguments for and against the use of the ECG in athletes’ pre-participation screening.
Arguments for and against the use of the ECG in pre-participation screening of young competitive athletes.
| For | Against |
|---|---|
| Availability | High false positive rate |
| The most common causes of SCD have characteristic findings | Lack of validated standardization criteria to define normal physiological changes |
| Higher sensitivity than history and physical examination | Not accurate for identifications of some causes of SCD |
| Italian experience showing a decline of SCD after ECG screening program implantation | Wide range of normal variants depending on demographic and sport-related characteristics |
| Early marker of SCD (frequently the first clinical event) | Lack of evidence from prospective studies |
| Improved diagnostic accuracy with modern standardized criteria | SCD is rare in young athletes and zero risk is unattainable |
| Lacks power and sensitivity of screening based on history and physical examination is low | Consumes healthcare resources that could be allocated to other and more common urgent needs |
| Identification of athletes with high risk for SCD leads to investigation of family members | Misinterpretation if physicians do not have expertise in sports cardiology |
| Cost-effective compared to history and physical examination | Inappropriate and expensive additional investigation |
| Interpretation of clinical history and physical examination are subjective and vary between physicians | Incorrect interpretation can lead to inappropriate disqualification |
| Low cost of mass screening (possibility of negotiating cost) | Cost-effectiveness remains to be determined |
| Ethical considerations: equal access should be provided to all sectors of society, not only to organizations with greater financial resources such as professional sports teams | Logistically impractical to implement for assessment of larger populations |
ECG: electrocardiogram; SCD: sudden cardiac death.
Several questions remain unanswered regarding the cardiac impact of exercise training and the assessment of competitive athletes.
The real prevalence of sudden cardiac death in athletesThe real prevalence of SCD in athletes remains unknown and the available data are controversial: values of 1:50 000, 1:100 000, and 1:20 000 have been proposed. Most studies are based on media reports, with obvious limitations. For example, a simple search for SCD in athletes in Portugal on www.google.com and the websites of the most widely read Portuguese newspapers (sports-related and generalist) reveals 46 cases in young or veteran athletes, 28 during competition, mainly soccer (n=13). This is unlikely to be the real number. The first death reported occurred in 1987; there were eight between 1997 and 2004, followed by the others, 12 occurring in 2004, the year of the case with the highest media profile in Portugal. This methodology is inaccurate and skewed. A systematic registry of all SCD cases is warranted and crucial for the epidemiological understanding of this tragic event.
Causes of sudden cardiac deathWithout knowing the causes of death in representative samples, it will be difficult to establish appropriate preventive protocols and screening programs. Analysis of SCD victims should include all the available resources for accurate etiological identification: meticulous post-mortem analysis, molecular autopsy, genetic tests and direct family history.
Normality and adaptive variantsIt is crucial to increase knowledge of normal and adaptive variant patterns of CV changes induced by exercise training at clinical, functional and morphological levels. Factors that should be taken into consideration include the impact of demographic characteristics such as ethnicity and extreme ages (from children to veterans), training-related issues, and the long-term effects of doping and intense exercise training.
Pre-participation screening programsIt is essential to define a single model for assessment of athletes that can be used in a sufficiently broad registry to draw definite conclusions on the specificity, sensitivity and overall cost-effectiveness of screening programs that would overcome the historical debate concerning the value of the ECG.
ConclusionsUnderstanding of cardiac physiological adaptation induced by continued intense exercise training is crucial for accurate assessment of competitive athletes. The overlap between these adaptations and cardiomyopathies poses a challenge: under-diagnosis of a disease may increase the risk of life-threatening events, and over-diagnosis can result in improper disqualification from competition. A high level of suspicion and inclusion of data from clinical history, physical examination and concomitant investigations are essential. Standardization of ECG interpretation could decrease the rate of false positives and allow early detection of potentially fatal CV abnormalities.
Conflicts of interestThe authors have no conflicts of interest to declare.