Takotsubo syndrome (TTS) is currently described as an acute and usually reversible form of systolic dysfunction of the left ventricle, which more frequently affects postmenopausal women after a stressful emotional event. Although TTS is a rare condition in premenopausal women, in recent years, the number of reported cases has increased. This manuscript reports the first case of a TTS several months after delivery in a 22-year-old woman during lactation. It may also emphasize the role of estrogens in the disease pathogenesis.
A síndrome de Takotsubo (STT) é presentemente descrita como uma forma aguda e geralmente reversível de disfunção sistólica do ventrículo esquerdo, que afeta mais frequentemente mulheres pós-menopáusicas após um evento emocionalmente stressante. Não obstante o STT ser uma entidade rara em mulheres pré-menopáusicas, nos últimos anos tem aumentado o número de casos descritos. Este artigo relata o primeiro caso de STT numa mulher de 22 anos durante o período de lactação, vários meses após o parto. Poderá, ainda, enfatizar o papel dos estrogénios na patogénese da doença.
Takotsubo syndrome (TTS) is currently described as an acute and usually reversible form of systolic dysfunction of the left ventricle, which most frequently affects postmenopausal women after a stressful emotional event.1–4 Catecholamines appear to be in the center of the pathogenesis, as the increased levels and reduced reuptake lead to catecholamine toxicity in the cardiomyocytes via occupation of adrenergic receptors. It also leads to coronary microcirculation dysfunction and myocardial stunning, through increased oxygen demand and oxidative stress.1,2,4 Additionally, the reduced levels of estrogen exacerbate the responses of central neurons and cardiac cells, which possibly attenuates some cardioprotective mechanisms.4,5 This hormone has a role in the activation of endothelial nitric oxide synthase, increasing the production of nitric oxide, and in the activation of a cardioprotective signaling cascade (including Akt and MAP kinases). Also, estrogen loss is associated with increased inflammatory cytokine expression and increased susceptibility to reactive oxygen species.1,6
Although TTS is a rare condition in premenopausal women, in recent years, the number of reported cases in these women has increased.7,8 We report the first case in literature, to our knowledge, of TTS in a 22-year-old woman several months after delivery, while breast feeding. These data may emphasize the role of estrogens in the disease pathogenesis.
Case reportWe present the case of a 22-year-old female patient, with a history of a seizure episode when she was 18 years-old, smoking habits and who was on oral contraceptives (desogestrel, a progestogen-only pill). She was also breastfeeding her five month old son (first-born child). A few minutes after an intense argument with her boyfriend, she had a syncope episode. Her relative described seizure-like movements, which lasted approximately five minutes. Afterwards, she was taken to the emergency department for evaluation.
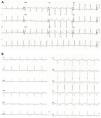
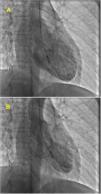
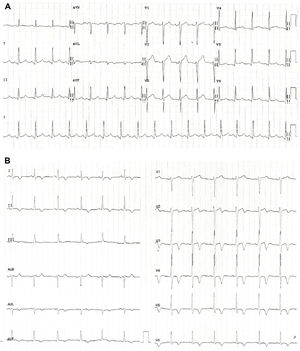
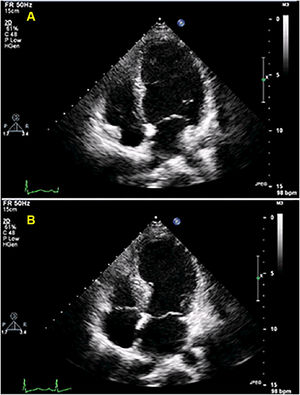
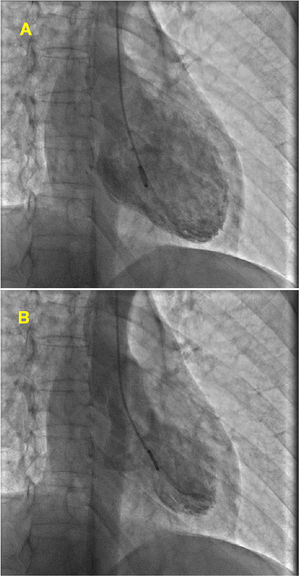
At admission, her heart rate was 105 bpm and blood pressure was 109/74 mmHg. Cardiac and pulmonary auscultation were normal. She denied having chest pain, respiratory distress, palpitations or recent infection. An electrocardiogram (ECG) was performed, revealing a discrete ST-segment elevation (<0.5 mm) in leads II, III, aVF and V2 to V6 and a slightly prolonged corrected QT interval (474 ms) (Figure 1A). The arterial blood gas analysis revealed compensated respiratory alkalosis without hypoxemia and the blood workup showed normal leukocyte count and C-reactive protein, elevated N-terminal brain natriuretic peptide (3300 pg/mL), positive troponin I (1.92 ng/mL, cut-off for myocardial infarction >0.1 ng/mL) and elevated D-dimer (1.2 μg/mL). A CT pulmonary angiogram was also performed but revealed no abnormalities. The echocardiogram showed akinesia of the midventricular and apical segments of all walls and hypercontractile basal segments, resulting in a severely depressed left ventricle ejection fraction (LVEF) (29%) (Figure 2). The right ventricle was undilated with a normal longitudinal function. No relevant valve disease or pericardial effusion were found. The patient was then transferred to the intensive cardiac care unit. The next day, the ECG showed a biphasic T wave in lead V2 and negative T waves in leads I, II, III, aVF, aVL and V3 to V6 (Figure 1B). A coronary angiography was performed and revealed no significant lesions of the epicardial coronary arteries. The ventriculography, however, showed an apical ballooning ventricle with a moderately depressed LVEF (Figure 3).
(A) Electrocardiogram at admission revealing a discrete ST-segment elevation in leads II, III, aVF and V2 to V6 and a slightly elevated corrected QT interval; (B) Electrocardiogram performed one day after admission showing a biphasic T wave in lead V2 and negative T waves in leads I, II, III, aVF, aVL and V3 to V6.
The differential diagnoses considering the systolic dysfunction were myocardial infarction with non-obstructive coronary arteries (MINOCA), myocarditis, peripartum cardiomyopathy (PPCM) and Takotsubo syndrome (TTS). She initiated therapy with bromocriptine, carvedilol, captopril and spironolactone.
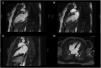
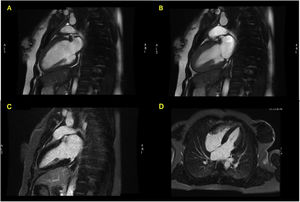
During in-hospital stay, she remained clinically and hemodynamically stable. The echocardiogram performed five days after admission showed an improvement in LVEF and wall motion abnormalities. A cardiac magnetic resonance (CMR) was also performed (seven days after admission), revealing a non-dilated LV, with normal LVEF (55%), no wall motion abnormalities and absence of late gadolinium enhancement (LGE) (Figure 4). The patient was also evaluated by a consultant neurologist and underwent an electroencephalogram and a magnetic resonance imaging of the brain (brain-MRI), which were both normal. She was discharged with bisoprolol 5 mg qd and referred to the cardiology outpatient clinic.
Cardiac magnetic resonance Imaging (Signa HDx 1.5T, GE Healthcare®) showing preserved ejection fraction and absence of late gadolinium enhancement. (A) FIESTA Cine in two-chambers view, diastole; (B) FIESTA Cine in two-chambers view, systole; (C) four-chambers view, after late gadolinium enhancement; (D) two-chambers view, after late gadolinium enhancement.
The follow-up echocardiogram performed two months after discharge showed preserved LVEF. As for the ECG, the QT interval was normal and repolarization abnormalities had disappeared.
DiscussionThis report describes a breastfeeding 22-year-old woman who had a syncope episode after a stressful emotional event. The workup found an apical ballooned left ventricle with severe systolic dysfunction, from which she rapidly recovered a week later.
Myocarditis is an unlikely hypothesis to explain this clinical picture, as it lacks a plausible epidemiologic context and epicardial late gadolinium enhancement (LGE). MINOCA is also a remote hypothesis, according to the low rise in troponin and the extension of the affected myocardium, plus the absence of subendocardial or transmural LGE.9
Peripartum cardiomyopathy, a rare condition of unclear etiology, which is possibly related to a cardiotoxic prolactine derivative that leads to systolic dysfunction, could be a plausible diagnostic hypothesis.10,11 However, 75% of the cases happen in first month postpartum, and the recovery of the diffuse hypokinesia (rather than apical ballooning) takes place after several months, with a complete normalization of LVEF in a reduced number of patients.8,10,12 Also, from the recognized risk factors for PPCM, the patient only presents smoking habits (multiparity, African American ethnicity, smoking, diabetes, pre-eclampsia, advanced age and teenage pregnancy).10,13
Takotsubo syndrome seems to be the most likely diagnosis, and this hypothesis is supported by several factors: the patient is of female gender; it was preceded by a stressful emotional event; no ST-segment depression and a slightly prolonged QT interval; the lack of correlation between a low maximum troponin level and the extension of affected myocardium; the ampullary-shaped ventricle and the rapid recovery of the systolic dysfunction; plus the absence of LGE on CMR.2,14
Despite the apparent typical clinical picture of TTS, the occurrence of syncope is an uncommon finding (7.7% of cases in a multicenter registry15) that could be explained by: a) transient dysrhythmia, with associated cerebral hypoperfusion and subsequent seizure-like movements2; b) seizures in an undiagnosed epilepsy patient. Several authors report association between seizures/status epilepticus and concomitant TTS, proposing a heart-brain interaction in TTS.1 Previous data indicate that patients suffering from status epilepticus present high catecholamine plasma levels (norepinephrine due to generalized sympathetic activation and epinephrine supposedly due to adrenal activation). Therefore, seizure activity may trigger conditions that induce TTS.16,17 Despite a normal EEG and brain-MRI, those findings do not firmly exclude an epilepsy diagnosis. Therefore, we can hypothesize that a concomitant physical trigger (seizure) could have also been present – in addition to a clearly identified emotional trigger.
Takotsubo syndrome is an uncommon condition in women of reproductive age but the number of affected women seems to be increasing. In a systematic review, Citro et al. reported 15 cases of TTS in postpartum women.8 None of the reported cases presented this condition 40 days after delivery and, to our knowledge, no TTS cases have been reported during the lactation period. In breast feeding women, high prolactin levels and consequent inhibition of gonadal axis could favor the disease mechanism, as the low estrogen levels lead to an impaired myocardial protection, becoming more susceptible to catecholamine toxicity.4,5,18
We believe that this case report could add relevant information about TTS in premenopausal women, as the hormonal state during lactation may favor the condition, emphasizing the role of estrogens in the pathogenesis of Takotsubo syndrome.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Dr. Agostinho Caeiro, Dr. Renato Fernandes, Dr. Tereza Veloso, Dr. João Pais and Dr. Mafalda Carrington for their support and availability.