Remote monitoring (RM) is a safe and effective alternative to in-office conventional follow-up.
ObjectiveWe aimed to evaluate patient satisfaction with RM and its impact on healthcare resources in a population with cardiac implantable electronic devices.
MethodsRandomized, pragmatic, open-label controlled trial, with adult wearers of implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy with ICD (CRT-D), eligible for the CareLink® system. Patients newly implanted or with previous conventional follow-up were randomized to RM or conventional follow-up (control), and followed for 12 months, according to the centers’ practice. The number of in-office visits and adverse events were compared between groups. Patient and healthcare professionals’ satisfaction with RM were described.
ResultsOf the 134 randomized patients (69 RM; 65 control, aged 60±13 years), 80% were male, 23% employed, 72% ICD wearers and 54% newly implanted. Most patients (70%) reported travel costs less than 15€/visit, and 46% daily routine interference with in-office visits. Median physician/technician time with patient was 15 min/15 min, per in-office visit. Excluding baseline and final visits, control patients had more in-office visits in total: median 1 vs. 0, p<0.001. In 81% of the in-office visits, no clinical measures were taken. There were 10 adverse events, with no differences between groups. At the final visit, 95% of RM patients considered RM easy/very easy to use, and would all prefer to maintain RM and recommend it to others. All professionals found the CareLink website easy/very easy to use and were satisfied with transmission data.
ConclusionsIn a Portuguese population with ICD and CRT-D, RM safely reduced the burden of in-office visits, with high levels of satisfaction among patients and healthcare professionals.
A monitoração remota (MR) é uma alternativa segura e eficaz ao acompanhamento convencional em consulta hospitalar.
ObjetivoAvaliar a satisfação dos doentes e o impacto nos recursos de saúde dum programa de MR numa população de portadores de dispositivos eletrónicos cardíacos implantáveis.
MétodosEnsaio clínico randomizado, aberto, controlado, com adultos portadores de cardioversor-desfibrilhador implantável (CDI) ou sistema de ressincronização cardíaca com CDI (CRT-D) elegíveis para MR com sistema CareLink®. Os doentes com implantação recente ou com acompanhamento convencional prévio foram randomizados para MR ou seguimento convencional (controle) e acompanhados por 12 meses, de acordo com a prática dos centros. O número de visitas hospitalares em consulta e os eventos adversos foram comparados entre os grupos. Foi analisada a satisfação dos doentes e profissionais de saúde relativamente à utilização da MR.
ResultadosDos 134 doentes randomizados (69 MR; 65 controle, idade 60 ± 13 anos), 80% eram do sexo masculino, 23% estavam empregados e ativos, 72% eram portadores de CDI e 54% tinham implantação recente. A maioria dos doentes (70%) reportou custos de viagem <15€/visita hospitalar e 46% referiram interferência na rotina diária devido às consultas hospitalares presenciais. O tempo mediano do doente com o médico/cardiopneumologista foi de 15 min/15 min por consulta hospitalar. Excluindo as consultas inicial e final, o grupo controle teve mais visitas hospitalares no total: mediana 1 versus 0, p<0,001. Em 81% das consultas presenciais não foi tomada medida clínica adicional. Houve 10 eventos adversos, sem diferenças entre os grupos. Na avaliação final, 95% dos doentes com MR consideraram a sua utilização fácil ou muito fácil e todos prefeririam manter a MR e recomendá-la a outros. Todos os profissionais de saúde acharam a plataforma CareLink fácil ou muito fácil de usar e revelaram satisfação com os dados das transmissões.
ConclusãoNuma população portuguesa com CDI e CRT-D, a MR reduziu de forma segura a carga de consultas hospitalares presenciais, com elevados níveis de satisfação dos doentes e profissionais de saúde.
Remote monitoring (RM) of cardiac implantable electronic devices (CIEDs) is a safe and effective alternative to conventional follow-up based only on ambulatory clinical visits.1,2 During the last decade, RM evolved to active transmission of patient- and device-triggered alerts, thus reducing the time to diagnosis and treatment.3 However, most clinical trials of RM failed to clearly demonstrate its advantage regarding patient outcomes.4–6 The type of CIED, previous patient experience with clinical follow-up, and the physicians’ perception of RM workload can compromise effective RM implementation.3–5 Patient satisfaction and impact on health resources should also be confirmed at a local level, since sociodemographic, economic and cultural aspects may influence these outcomes.4,7
The Portuguese Research on Telemonitoring with CareLink (PORTLink) study evaluated whether the use of the Medtronic CareLink® system for RM of subjects with implantable cardioverter-defibrillators with (CRT-D) or without (ICD) cardiac resynchronization therapy devices improves the follow-up efficiency, in particular with regards to patient satisfaction and the use of resources, when compared with exclusively in-office follow-up and independently of patient previous experience of conventional follow-up.
MethodsStudy designThe PORTLink study was a pragmatic, randomized, controlled, open-label, multicenter study conducted in six Portuguese reference centers for CIEDs. The study was approved by the ethics committees of each study site and by the Portuguese Authority on Data Protection. All patients provided their written informed consent to participate (ClinicalTrials.gov: NCT03125382).
The rationale, design and protocol have been described previously.8 In brief, included patients were ≥18 years-old, implanted with a Medtronic ICD or CRT-D and eligible to use the CareLink® service for RM of CEID. Recruitment took place between April 2012 and May 2015, and participants were to be followed for 12 months. Upon enrollment and registry on the study database, participants were assigned on a 1:1:1:1 basis (simple randomization concealed to the investigators) into the following groups: recently implanted starting follow-up on the RM protocol (group A – RM) or starting conventional follow-up (group B – control); with previous experience on conventional follow-up changing to the RM protocol (group C – RM) or without changing to the RM protocol (group D – control).
Study endpointsClinical forms and patient questionnaires were completed during ambulatory visits and after each remote data transmission and collated into the study database. The study primary endpoints were the proportion of patients satisfied with the monitoring protocol, the resources consumed (number of outpatient clinic visits and associated costs from the patient standpoint), the healthcare professionals (HCP) satisfaction with the CareLink service, and the number of adverse events during a 12-month period.
Health-related quality of life was measured with an adapted version of the SF-12 questionnaire, a 12-item generic measure derived from the Short-Form 36.9 It evaluates physical functioning, limitations due to physical health problems, bodily pain, energy/fatigue, social functioning, limitations due to emotional problems, and psychological distress and well-being. Physical component summary (PCS) and mental component summary (MCS) measures were estimated, with higher scores indicating better health status.
Mental health wellbeing was measured with the Hospital Anxiety and Depression Scale (HADS), which consists of a series of 14 questions, seven related to anxiety (HAD-A) plus another seven questions related to depression (HAD-D).10,11 Scores of 0-7 for the two subscales were regarded as normal, scores of 8-10 suggested the presence of a mood disorder and scores ≥11 suggested a probable mood disorder.12
Patient satisfaction with healthcare provided at the hospital was evaluated with a five points Likert scale question, with 1 and 5 indicating the lowest and highest levels of satisfaction, respectively.
Statistical analysisThe statistical methods and sample size were defined on the basis of the trial's objectives and assuming a confidence level of 95% (two-tailed) and 80% power.5 The main clinical and sociodemographic variables are summarized at baseline, as mean – standard deviation (SD), median (25th-75th percentiles) and counts (percentages) for continuous and categorical variables, respectively. Normality was assessed with the Kolmogorov-Smirnov and Shapiro–Wilk tests. Study groups were compared using the Student's t-test or non-parametric Mann-Whitney U test, for continuous variables and with Chi-square test or Fisher's exact test, as appropriate to categorical variables. Within each group, the nonparametric Wilcoxon rank-sum test for paired samples was used to determine the statistical significance of changes on anxiety, depression and SF-12 PCS and MCS scores. Poisson regression models were used to examine the effect of RM on the number of in-office visits, after adjusting for study center and other variables that showed statistically significant differences at baseline. Changes in HADS anxiety and depression scores, SF-12 PCS and MCS and satisfaction with clinical care were evaluated by analysis of covariance (ANCOVA) models, which included the RM vs. control group as an indicator variable and adjusted for the baseline values and previous follow-up experience. The statistical analysis was conducted using the software IBM SPSS® Statistics 25.
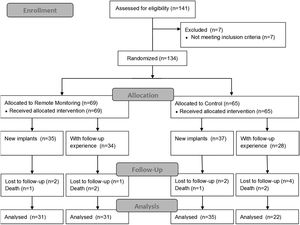
ResultsStudy participants at baselineA total of 141 patients were enrolled from six participating centers, from which 134 were randomized: 65 were followed-up with routine in-site medical appointments and 69 received the RM protocol (Figure 1). The enrollment rate ranged from 1 to 12 participants per month. The mean ± SD follow-up by patient was 15.1±4.2 months.
Overall, 79.9% of the randomized patients were men, with a median educational level of four years (corresponding to the elementary level in Portugal) and, at baseline, median age was 60 years (mean ± SD: 59.9±13.0 years) and 22.7% were employed (Table 1). Regarding clinical characteristics, the majority of participants had an ICD (71.8%), presented normal right ventricle function (62.4%), less than one quarter (22.4%) had cardiac symptoms and about a third (32.1%) presented spontaneous arrhythmic events.
Baseline characteristics of randomized participants.
| Totaln=134 | RMn=69 | Controln=65 | p-value | |
|---|---|---|---|---|
| Age (years) | 60 [53–69] | 60 [54–69] | 60 [52–69] | 0.79† |
| Men | 107 (79.9) | 55 (79.7) | 52 (80.0) | 0.97* |
| Educational level (years) | 5 [4–9] | 4 [4–9] | 6 [4–11] | 0.13† |
| Employed | 30 (22.7) | 17 (24.6) | 13 (20.0) | 0.52* |
| Time since first implant (months) | 5 [2–28] | 5 [2–31] | 6 [2–28] | 0.99† |
| Implantable cardioverter-defibrillator | 96 (71.8) | 51 (73.9) | 45 (69.2) | 0.55* |
| NYHA functional classa | 0.39* | |||
| I | 34 (31.2) | 16 (27.6) | 18 (35.3) | |
| II | 58 (53.2) | 32 (55.2) | 26 (51.0) | |
| III/IV | 17 (15.6) | 10 (17.2) | 7 (13.7) | |
| Not applicable | 25 | 11 | 14 | |
| Left ventricle ejection fraction | 29 [24–35] | 29 [24–35] | 29 [24–34] | 0.77† |
| Right ventricle function normal | 83 (87.4) | 51 (96.2) | 32 (76.2) | 0.004* |
| Not applicable | 37 | 16 | 21 | |
| Any cardiac symptoms | 30 (22.4) | 17 (24.6) | 13 (20.0) | 0.52* |
| Any spontaneous arrhythmic events | 43 (32.1) | 27 (62.8) | 16 (37.2) | 0.07* |
| Previous intervention/surgery | 52 (40.6) | 26 (40.0) | 26 (41.3) | 0.88* |
| Any other cardiovascular risk factors | 97 (75.8) | 51 (76.1) | 46 (75.4) | 0.93* |
| Travel to hospital – distance (km) | 16 [8–30] | 17 [8–30] | 15 [8–32] | 0.80† |
| Travel to hospital – time spent per visit (min) | 30 [20–60] | 30 [20–60] | 30 [15–60] | 0.38† |
| Travel to hospital – costs per visit | 0.84* | |||
| Up to 5€ | 46 (34.6) | 23 (33.3) | 23 (35.9) | |
| (5-15€) | 47 (35.3) | 26 (37.7) | 21 (32.8) | |
| >15€ | 40 (30.1) | 20 (29.0) | 20 (31.3) | |
| In-office visits disturb patient daily life | 60 (45.5) | 31 (44.9) | 29 (46.0) | 0.90* |
| Patients accompanied during this visit | 68 (51.9) | 35 (51.5) | 33 (52.4) | 0.92* |
| (yes) In-office visits disturb caregiver daily lifeb | 49 (72.1) | 25 (71.4) | 24 (72.7) | 0.91* |
| Time spent by patient at hospital due to visit (min) | 90 [55–138] | 95 [60–140] | 80 [50–110] | 0.12† |
| In-office time with physician (min) | 15 [10–20] | 15 [10–20] | 15 [10–20] | 0.39† |
| In-office time with technician (min) | 15 [10–20] | 15 [10–20] | 15 [10–20] | 0.96† |
| Any action after review of clinical data | 17 (12.8) | 10 (58.8) | 7 (41.2) | 0.54* |
| Visits and hospitalizations during previous yearc | n=62 | n=34 | n=28 | |
| At least one scheduled in-office visit | 59 (96.7) | 34 (100.0) | 25 (92.6) | 0.19** |
| (yes) number per patient | 2 [2–2] | 2 [2–2] | 2 [1–2] | 0.86† |
| At least one unscheduled in-office visit | 10 (16.7) | 7 (21.2) | 3 (11.1) | 0.49** |
| (yes) number per patient | 1 [1–2] | 2 [1–3] | 1 [1–1] | 0.18† |
| Any emergency visit | 23 (37.7) | 16 (47.1) | 7 (25.9) | 0.09* |
| (yes) number per patient | 2 [1–2] | 1.5 [1–2] | 2 [1–4] | 0.34† |
| Any hospitalization >24 h | 19 (31.1) | 12 (35.3) | 7 (25.9) | 0.43* |
| (yes) number per patient | 2 [1–2] | 2 [1–2] | 1 [1–3] | 0.77† |
NYHA: New York Heart Association; RM: remote monitoring.
Values are number (%) or median [25th-75th percentiles], except otherwise mentioned.
Most patients (70%) reported up to 15€ on travel costs to the center, and 46% stated that the in-office visits disrupted their daily routine. Patients spent a median time with the physician of 15 min (mean ± SD: 15.6±6.2 min) plus 15 min (mean ± SD: 16.0±6.0 min) with technicians, per in-office visit. In 81% baseline visits, no actions were required after review of clinical data. Overall, the most frequent actions were reprogramming of device parameters (in 12 visits), change of medications (in six visits) and request for additional examinations (in two visits).
There were no statistically significant differences between RM and control patients, except for the higher proportion of RM patients with normal right ventricle function (96.2% vs. 76.2%; p-value=0.004). Supplemental Table S1 displays the comparison of RM vs. control patients, according to newly implanted vs. experienced patients. Within the newly implanted patients, a higher proportion of RM patients had normal right ventricle function (93.1% vs. 68.0%; p-value=0.032). Regarding patients with previous follow-up experience, a higher proportion of RM patients were classified as NYHA functional class II/III (75.9% vs. 47.6%; p-value=0.040).
Follow-up evaluationParticipants in the control groups had more in-office visits (74 vs. 28 visits in RM groups), corresponding to a median number of 1 vs. 0 visits per patient (p<0.001) – Table 2. Based on the estimates of the Poisson regression models, participants in the RM had a 67.8% decrease of scheduled visits (Exp(β)=0.322 (95% CI, 0.204-0.508); p<0.001), after adjusting for study center. For patients with an evaluation of right ventricular function (n=95), participants under RM had a 73.1% decrease in scheduled visits (Exp(β)=0.269 (95% CI, 0.149-0.488); p<0.001), after adjusting for changes in the right ventricular function and study center.
In-office visits during follow-up.
| Totaln=134 | RMn=69 | Controln=65 | p-Value | |
|---|---|---|---|---|
| Follow-up duration, months | 13 [11–18] | 13 [11–18] | 13 [12–16] | 0.90† |
| At least one scheduled in-office visit | 73 (54.5) | 20 (29.0) | 53 (81.5) | <0.001* |
| (yes) number per patient | 1.3; 1 [1–2] | 1.3; 1 [1–2] | 1.3; 1 [1–2] | 0.69† |
| At least one unscheduled in-office visit | 5 (3.7) | 2 (2.9) | 3 (4.6) | 0.67** |
| (yes) number per patient | 1.0; 1 [1–1] | 1.0; 1 [1–1] | 1.0; 1 [1–2] | >0.99† |
| In-office visits per patienta | ||||
| All | 0.8; 1 [0–1] | 0.4; 0 [0–1] | 1.1; 1 [1–1] | <0.001† |
| Scheduled | 0.7; 1 [0–1] | 0.4; 0 [0–1] | 1.1; 1 [1–1] | <0.001† |
| Unscheduled | 0.0; 0 [0–0] | 0.0; 0 [0–0] | 0.1; 0 [0–0] | 0.58† |
| Total number of in-office visits | 102 | 28 | 74 | - |
| Type of in-office visitsb | 0.66* | |||
| Scheduled | 96 (94.1) | 26 (92.9) | 70 (94.6) | |
| Any action after review of clinical datac | 12 (12.5) | 2 (7.7) | 10 (14.3) | |
| Request additional examinations, n | 7 | 1 | 6 | |
| Device reprogramming, n | 4 | 1 | 3 | |
| Change of medication, n | 3 | 0 | 3 | |
| Hospitalization – ablation, n | 1 | 0 | 1 | |
| Unscheduled | 6 (5.9) | 2 (7.1) | 4 (5.4) | |
| Any action after review of clinical datac | 3 (50.0) | 1 (50.0) | 2 (50.0) | |
| Device reprogramming, n | 2 | 1 | 1 | |
| Change of medication, n | 1 | 0 | 1 | |
RM: remote monitoring.
Values are number (%) and mean; median [25th-75th percentiles], except otherwise mentioned.
There were no statistically significant differences between groups regarding the number of unscheduled visits that occurred in 2.9% RM and 6.2% control patients. During follow-up, the majority of scheduled visits required no action after review of clinical data, while these actions were required in half of the unscheduled — in both groups.
Remote transmissions and patient satisfaction with remote monitoringPatients in the RM groups had a total of 252 remote transmissions, from which 97 (38.6%) were unscheduled (Table 3). The majority of transmissions were assessed by technicians, and only cases with occurrence of alerts were reviewed by the physician. Patients presented spontaneous arrhythmic episodes during 29% transmissions and new clinical conditions were identified after the revision of nine transmissions. For the vast majority of transmissions (94.8%, n=239) additional clinical actions were not initiated, while rescheduling of in-office visits occurred for six transmissions. All HCP reported the CareLink website as easy to use and were satisfied or highly satisfied with the quality of data transmissions. For the majority of patients, HCP reported no difficulties with RM system (n=50 patients). However, bureaucratic aspects (n=13, all reported by one center), time constrains (n=3) and few technical limitations were mentioned, namely: “does not allow testing” (n=1); “it is not possible to change device programming” (n=1); “sometimes, the data are only available on the website a few days after transmission” (n=1).
Changes of anxiety and depression, quality of life and satisfaction with clinical care.
| Totaln=134 | RMn=69 | Controln=65 | p-Value | |
|---|---|---|---|---|
| HADS anxiety score | ||||
| Baseline | 7 [4–10] | 7 [4–9] | 7 [4–10] | 0.90† |
| Normal | 77 (58.8) | 40 (58.8) | 37 (58.7) | 0.72* |
| Borderline | 28 (21.4) | 16 (23.5) | 12 (19.0) | |
| Abnormal | 26 (19.8) | 12 (17.6) | 14 (22.2) | |
| Final | 6 [4–8] | 6 [4–8] | 6 [3–8] | 0.42† |
| Normal | 85 (72.6) | 45 (72.6) | 40 (72.7) | 0.35* |
| Borderline | 15 (12.8) | 10 (16.1) | 5 (9.1) | |
| Abnormal | 17 (14.5) | 7 (11.3) | 10 (18.2) | |
| Change | 0 [-3 to 1] | 0 [-3 to 1] | 0 [-3 to 0] | 0.53‡ |
| p-value within group | 0.002 | 0.023 | 0.045 | - |
| HADS depression score | ||||
| Baseline | 4 [2–7] | 4 [2–8] | 4 [2–7] | 0.64† |
| Normal | 100 (76.3) | 50 (73.5) | 50 (79.4) | 0.73* |
| Borderline | 21 (16.0) | 12 (17.6) | 9 (14.3) | |
| Abnormal | 10 (7.6) | 6 (8.8) | 4 (6.3) | |
| Final | 4 [2–7] | 4 [2–8] | 5 [1–7] | 0.93† |
| Normal | 90 (76.9) | 46 (74.2) | 44 (80.0) | 0.60* |
| Borderline | 12 (10.3) | 8 (12.9) | 4 (7.3) | |
| Abnormal | 15 (12.8) | 8 (12.9) | 7 (12.7) | |
| Change | 0 [-2 to 1] | 0 [-2 to 1] | 0 [-2 to 1] | 0.48‡ |
| p-value within group | 0.57 | 0.53 | 0.85 | - |
| Physical component score | ||||
| Baseline, mean ± sd | 42.6±8.1 | 41.6±7.7 | 43.6±8.3 | 0.16§ |
| Final, mean ± sd | 42.0±7.7 | 42.5±7.5 | 41.3±7.9 | 0.44§ |
| Change | -0.6 [-5.2 to 3.2] | 0.0 [-4.5 to 4.9] | -1.1 [-8.1 to 2.6] | 0.18† |
| Change, mean ± sd | -0.7±8.9 | 0.6±9.3 | -2.2±8.2 | 0.23‡ |
| p-value within group | <0.001 | <0.001 | <0.001 | - |
| Mental component score | ||||
| Baseline, mean ± sd | 42.4±9.6 | 43.1±9.6 | 41.7±9.7 | 0.40§ |
| Final, mean ± sd | 42.0±8.2 | 41.4±9.2 | 42.8±6.9 | 0.36§ |
| Change | 0.0 [-5.8 to 6.4] | 0.0 [-9.5 to 5.6] | 0.4 [-3.9 to 7.5] | 0.26† |
| Change, mean ± sd | 0.3±11.0 | -0.7±11.4 | 1.4±10.4 | 0.28‡ |
| p-value within group | <0.001 | <0.001 | <0.001 | - |
| Patient satisfaction with clinical care | ||||
| Baseline | 0.64*b] | |||
| Very satisfied | 74 (56.1) | 40 (58.0) | 34 (54.0) | |
| Satisfied | 56 (42.4) | 28 (40.6) | 28 (44.4) | |
| Neither satisfied or unsatisfied | 2 (1.5) | 1 (1.4) | 1 (1.6) | |
| Unsatisfied/very unsatisfied | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Final visit | 0.91*b] | |||
| Very satisfied | 61 (51.3) | 32 (50.8) | 29 (51.8) | |
| Satisfied | 56 (47.1) | 30 (47.6) | 26 (46.4) | |
| Neither satisfied or unsatisfied | 2 (1.6) | 1 (1.6) | 1 (1.8) | |
| Unsatisfied/very unsatisfied | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
HAD: Hospital Anxiety and Depression Scale; RM: remote monitoring.
Values are number (%) or median [1st-3rd quartiles], except otherwise mentioned. RM, remote monitoring.
The most frequent benefits from the HCP perspective, as reported by patient, were greater safety for the patient (n=34), early detection of changes (n=8), greater comfort for the patient (n=20), closer proximity to the patient (n=7), faster evaluation of transmission data (n=4), easy interface (n=25), greater convenience for the center (n=14), greater schedule flexibility/scheduling facility (n=25), possibility of team discussion without having the patient present (n=8), and possibility of sending transmissions and sharing information with other professionals/centers (n=3).
At the end of follow-up, all RM patients (n=62) would prefer to continue with remote transmissions and have in-office visits only when scheduled by the medical doctor (compared to only having in-office visits, even if more frequent) and all would recommend it to another person with a cardiac device. All patients were satisfied (55.7%) or highly satisfied (44.3%) with the RM experience, and the majority (95%) considered RM easy or very easy to use at home. When asked if the RM affected their emotional status, 36.1% of the patients reported a positive effect, due to feeling safer (n=13), reassured (n=9), calm (n=3) and having to travel less (n=2). There were no statistically significant differences between RM patients newly implanted or with previous conventional follow-up (Table S2).
Hospital Anxiety and Depression Scale and quality of lifeDuring follow-up, a statistical significant improvement in HADS anxiety score was observed within both RM and control groups, with no statistically significant differences between groups (Table 4). Physical and mental component scores also showed no statistically significant differences between RM and control groups, despite changes being statistically significant within groups – in the RM group, the physical component score increased slightly while the mental component score decreased slightly. There were no statistically significant changes in patient satisfaction with the care received at the clinic.
Remote transmissions during follow-up and healthcare professionals’ opinion regarding CareLink® system.
| Total RM (n=69) | RM – new implants (n=35) | RM – with follow-up experience (n=34) | |
|---|---|---|---|
| Patients with at least one remote transmission | 63 (91.3) | 30 (85.7) | 33 (97.1) |
| Patients with wireless CareLink Monitor | 61 (88.4) | 35 (100.0) | 26 (76.5) |
| Number of transmissions by patient | 3 [2–4] | 3 [2–5] | 3 [3–4] |
| Total number of remote transmissions, na | 252 | 124 | 128 |
| Scheduled | 154 (61.3) | 67 (54.1) | 87 (68.5) |
| Unscheduled | 97 (38.6) | 57 (46.0) | 40 (31.5) |
| Reasons for unscheduled remote transmissions | |||
| AT/AF daily burden | 4 (4.1) | 3 (5.3) | 1 (2.5) |
| Battery alert | 2 (2.1) | 0 (0.0) | 2 (5.0) |
| Biventricular pacing <90% | 9 (9.3) | 9 (15.8) | 0 (0.0) |
| Capture management warning | 43 (44.3) | 21 (36.8) | 22 (55.0) |
| NSVT | 5 (5.2) | 1 (1.8) | 4 (10.0) |
| Patient initiative (no symptoms) | 11 (11.3) | 8 (14.0) | 3 (7.5) |
| Patient initiative (symptoms) | 3 (3.1) | 1 (1.8) | 2 (5.0) |
| Possible fluid accumulation | 6 (6.2) | 6 (10.5) | 0 (0.0) |
| Requested by center after unsuccessful automatic transmission | 4 (4.1) | 1 (1.8) | 3 (7.5) |
| VT/VF episode | 8 (8.2) | 6 (10.5) | 2 (5.0) |
| Other | 2 (2.1) | 1 (1.8) | 1 (2.5) |
| Did this unscheduled transmission | |||
| Prevented an emergency visit at the hospital? | 1 (1.0) | 1 (1.8) | 0 (0.0) |
| Prevented an unscheduled in-office visit? | 5 (5.2) | 4 (7.0) | 1 (2.5) |
| Detection of spontaneous arrhythmic eventsa,b | 73 (29.1) | 37 (29.8) | 36 (28.3) |
| VT/VF | 11 | 5 | 6 |
| NSVT | 59 | 25 | 34 |
| AT/AF | 12 | 11 | 1 |
| Any action after review of transmission dataa,b | 15 (6.0) | 7 (5.6) | 8 (6.3) |
| Anticipation of the next in-office visit | 6 | 4 | 2 |
| Schedule of remote transmission within one month | 4 | 2 | 2 |
| Request of new remote transmission | 3 | 0 | 3 |
| Phone contact to evaluate patient condition | 2 | 1 | 1 |
| Remote transmissions reviewed by medical doctora | 7 (2.8) | 5 (4.0) | 2 (1.6) |
| Time spent during review of transmission data (min) | |||
| Scheduled | 7 [5–10] | 5 [4–8] | 10 [5–10] |
| Unscheduled | 4 [2–7] | 4 [2–5] | 5 [2–7] |
| Adequate remote transmission data (i.e., provides same information as an in-office visit)a | 251 (100.0) | 124 (100.0) | 127 (100.0) |
| Overall satisfaction with the CareLink websitea | |||
| Very satisfied with transmission data | 178 (71.2) | 98 (79.7) | 80 (63.0) |
| Satisfied with transmission data | 72 (28.8) | 25 (20.3) | 47 (37.0) |
| How easy it was to navigate the CareLink websitea | |||
| Very easy | 187 (74.5) | 102 (82.3) | 85 (66.9) |
| Easy | 64 (25.5) | 22 (17.7) | 42 (33.1) |
Values are number (%) or median [1st-3rd quartiles], except otherwise mentioned. RM, remote monitoring.
There were six deaths (three patients from the RM groups and three patients with conventional follow-up) and seven serious adverse events reported in 10 patients (six patients with conventional follow-up). Two deaths were classified as non-sudden cardiac death – one RM patient due to acute pulmonary edema and one conventional patient due to pulmonary embolism. One patient with conventional follow-up died due to accelerated progression in chronic kidney disease. Three deaths (of which two were RM patients) were of non-cardiac cause. Other serious adverse events were: recurrent ventricular tachycardia (VT) episodes (two patients with conventional follow-up), atrial fibrillation with rapid ventricular response in one patient with conventional follow-up and acute decompensated heart failure of one RM patient. None of the deaths or adverse events were related to RM.
DiscussionThe daily impact of CIEDs is still a matter of concern for patients and caregivers. The use of RM has been recognized as being valuable in the follow-up of patients with these devices, by promoting a closer follow-up and improving outcomes.13
Our results demonstrated a statistically significant reduction in scheduled in-office visits and highlight patients’ satisfaction with RM. They reported feeling felt safer and reassured with this monitoring protocol. Moreover, the vast majority of RM transmissions did not require any additional action.
Previous studies have shown that RM is feasible in clinical practice, reducing the number of ambulatory scheduled visits, hospitalizations and emergency room visits and optimizing the use of healthcare resources.14–16 We observed a reduction of approximately one scheduled visit between baseline and final visit (i.e., after 12 months), and this effect would probably increase with a longer follow-up. On the other hand, a reduction of in-office visits in spite of the study might have occurred, as the number of scheduled visits reported by patients with previous follow-up experience was somewhat higher than the one observed during follow-up of the control group (median: 2 vs. 1). Even so, assuming that almost half of participants reported disruptions in their daily life by the in-office visits (and for their caregivers), that each appointment took about 30 min of HCP time and, for the patient, a median time at the hospital of 90 min plus a median travel time of 30 min (summing up to about 2.5 h of patient time), and that the large majority of in-office visits had no additional clinical action after review of CIED data, this RM reduction would translate into increased monitoring efficiency for both patients and HCP, as reported by others.6,16,17
Although no statistically significant effects of RM were observed in anxiety/depression, neither on patients’ quality of life or satisfaction with received clinical care, we observed high levels of patient satisfaction with RM, related to the perception of increased safety and less time-consuming and travel costs.14,18,19 Surprisingly, all patients preferred to continue with RM and have in-office visits only when scheduled by the medical doctor. Based on a previous Portuguese single-center study, we were expecting lower levels of RM acceptance and that the face-to-face contact was missed by some patients.20 Despite the learning curve of the RM system implementation, and that some technical issues may need more time to be resolved and workload to be suppressed, the participating HCP considered the RM website easy to use and reported being satisfied or highly satisfied with RM and quality of data transmissions.4,19,21,22
The safety of RM has been demonstrated in several studies, and especially in the large registry study with 69556 ICD and CRT-D patients (ALTITUDE study), where a 50% reduction in mortality was observed.23 In Portugal, a propensity score-matched cohort study of ICD patients concluded that RM was associated with a lower rate of a combined endpoint of hospital admission for heart failure or cardiovascular death, during a mean follow-up of 44 months.24 However, a meta-analysis of randomized controlled trials failed to clearly demonstrate the RM effect on reducing mortality and hospitalizations.3 In our study, two non-sudden cardiac deaths occurred, one in each group, and patients in the control group had more serious cardiac adverse events.
Most of the unscheduled remote transmissions were due to “capture management warning” and newly implanted patients had more events leading to unscheduled transmissions (e.g., biventricular pacing <90%, possible fluid accumulation and VT/ventricular fibrillation episodes). The unscheduled remote transmissions and visits did not translate into additional hospitalizations. Hence, our data suggest that RM is a safe alternative to conventional follow-up.
Some study limitations should be recognized. The study had several recruitment constraints, as it started during a severe economic crisis which impacted on the number of implanted cardiac devices, as well as the number of routinely scheduled in-office visits. The recruitment period exceeded the projected timeline and, despite the inclusion of six centers, it was not possible to achieve the defined sample size (n=50 per each of the four study groups). Nevertheless, since there were no major baseline differences between patients with or without previous experience of conventional follow-up, the analysis was maintained at the comparison of RM vs. conventional follow-up and adjusted for previous experience, when required. We also appreciate that the extended recruitment period comprehends a paradigm shift regarding the added-value of RM of CEID, mostly driven by the results of the ALTITUDE study.1,23 For that reason, we could not rule out the true effect of RM on the reduction of in-office visits in Portugal having been underestimated. Since we included only those patients with Medtronic® cardiac devices, the extrapolation of the study results to other RM systems should be cautious.4
The present global pandemic has highlighted the importance of telemedicine.18,25,26 The PORTLink study was one of the few randomized controlled trials evaluating the effect of RM of both ICD and CRT-D among patients with or without previous follow-up experience 8, and, to the best of our knowledge, the only one conducted in Portuguese centers. It also illustrates the scientific potential of pragmatic RCTs – additional study visits were not required and it was mostly based on routinely collected data – while providing robust real-world evidence of the added-value of RM in Portugal.
ConclusionRemote monitoring is a safe alternative to conventional follow-up of ICD and CRT-D wearers, reducing the number of in-office visits and the social impact for the patients. Healthcare professionals were highly satisfied with the RM protocol. Patients found RM easy to use and were highly satisfied with this protocol, favoring it as follow-up methodology.
Data sharing statementThe data underpinning this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data can be shared on reasonable request to the corresponding author.
FundingThe study was sponsored by Medtronic, with no role in data analysis (provided by Preventive Medicine and Public Health Institute) or interpretation of study results.
Conflicts of interestMário Oliveira is a member of the European Advisory Board of Medtronic. Mónica Silva is an employee of Medtronic Portugal. The other authors have no conflicts of interest to declare.
The authors acknowledge the PORTLink study investigators at the participating centers: Manuel Nogueira da Silva, Sofia Santos, Ricardo Pimenta, Manuel Brás, Ana Sofia Delgado (Centro Hospitalar Universitário Lisboa Central); Vitor Lagarto, Carla Roque, Sandra Santos, Paulo Costa, Ernesto Aranda (Centro Hospitalar Universitário do Porto); Marco Oliveira, Helena Gonçalves, Luís Adão, José Ribeiro, Elisabeth Santos, Joana Braga (Centro Hospitalar Vila Nova de Gaia/Espinho); Sílvia Ribeiro, Assunção Alves, Bernardete Rodrigues, Miguel Mariz, António Miguel Santos, Tiago Alves (Centro Hospitalar Alto Ave); Rui Candeias, Ilídio de Jesus, Luísa Segismundo, Patricia Baptista, Ana Santos (Centro Hospitalar do Algarve); João Madeira, Cláudia Lopes (Centro Hospitalar Setúbal).
The authors also acknowledge all participating patients, and Pedro Aguiar for the overview of the statistical analysis. The study was funded by Medtronic Portugal, Lda.