Pragmatic validated instruments are needed to assess quality of life (QoL) in the real-world care of heart failure (HF) patients. This study aimed to validate the Minnesota Living with Heart Failure Questionnaire (MLHFQ) in European Portuguese and to search for associations with other patient-reported outcomes (PROs).
MethodsThis is a single-center prospective study with two samples of HF patients, used for MLHFQ translation, cultural adaptation and validation. Validation sample included patients managed in a multidisciplinary HF clinic between February 29, 2016, and February 29, 2020.
ResultsThe validation sample included 294 HF patients, with a median age of 78 years, 53.1% female sex, 45.2% with reduced left ventricle ejection fraction (LVEF) and 43.9% with preserved LVEF. The principal component analysis identified three components, representing three groups of questions from the MLHFQ, each related to one domain and subscore: physical, emotional and social. The validated version showed a reliable general factor and good internal consistency. MLHFQ total score was associated with all the other variables studied. All the scores were strongly associated with NYHA functional class. Associations were found between the physical score and both the Borg fatigue score and the six-minute walking distance. Emotional score was associated with both anxiety and depression scores from Hospital Anxiety and Depression Scale.
ConclusionsThis study validated the MLHFQ in Portuguese and revealed the existence of three subscores. It enables the pragmatic assessment of the QoL of Portuguese HF patients, particularly of those with preserved LVEF, and future research concerning PROs.
Na prática clínica são necessários instrumentos validados e pragmáticos para avaliar a qualidade de vida (QdV) dos doentes com insuficiência cardíaca (IC). Este estudo tem como objetivo validar o Minnesota Living with Heart Failure Questionnaire (MLHFQ) para português e procurar associações com outros resultados reportados pelos doentes (PROs).
MétodosEste é um estudo prospetivo e observacional de um só centro, com duas amostras de doentes com IC, utilizadas para a tradução, adaptação cultural e validação do MLHFQ. A amostra de validação incluiu doentes integrados numa clínica multidisciplinar de IC entre 29/02/2016 e 29/02/2020.
ResultadosA amostra de validação incluiu 294 doentes, com uma mediana de idades de 78 anos, 53,1% do sexo feminino, 45,2% com fração de ejeção do ventrículo esquerdo (FEVE) reduzida e 43,9% com FEVE preservada. A análise do componente principal identificou 3 componentes, representando 3 grupos de questões do MLHFQ, cada um relacionado com um domínio e uma pontuação parcial: físico, emocional e social. A versão validada mostrou um fator geral fiável e boa consistência interna. A pontuação total do MLHFQ associou-se a todas as outras variáveis estudadas. Todas as pontuações se associaram fortemente à classe funcional de NYHA. Adicionalmente, foram encontradas associações entre a pontuação do domínio físico e a pontuação de cansaço na escala de Borg, assim como com a distância percorrida em 6 minutos. A pontuação do domínio emocional revelou associações com as pontuações de ansiedade e de depressão da Hospital Anxiety and Depression Scale.
ConclusõesEste estudo validou o MLHFQ em português e revelou a existência de 3 pontuações parciais. Torna assim possível a avaliação pragmática da QdV em doentes portugueses com IC, particularmente daqueles com FEVE preservada, e a investigação futura no âmbito dos PROs.
Quality of life (QoL) is a distinctive human aim only recently introduced as an important outcome and quality indicator for the management of patients with heart failure (HF).1,2 The World Health Organization defines QoL as “an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns.”3 This view reinforces the contemporary concept of QoL having a broad and multilayered scope, which should be considered when assessing it in the context of underlying diseases, such as chronic HF.4
HF is the leading cause of hospitalization in people over 65 years of age in developed countries.5 In Portugal, it affects approximately 770000 patients, with a prevalence of 16.54% in people aged ≥50 years.6,7
Traditionally, the primary endpoints of HF trials consisted of all-cause or cardiovascular mortality and/or HF hospitalizations. QoL and patient-reported health outcomes (PROs) have been viewed as “soft” endpoints, although they provide insight into treatment effects from the patient's perspective.8 In 2013, a clinical outcome endpoints consensus document in HF trials was published by the European Society of Cardiology (ESC), reporting symptoms and PROs as independent endpoints for HF trials. The document stated the need for development of validated and simpler instruments sensitive to changes in health status over time.1
There are several validated instruments to assess QoL in patients with HF. The two most used questionnaires are the Minnesota Living with Heart Failure Questionnaire (MLHFQ)9 and the Kansas City Cardiomyopathy Questionnaire (KCCQ).10 The use of these instruments, however, remains largely limited to clinical trials.11 They are both time-intensive, and their current use in real-world care is very limited.2,12 Other authors identified the MLHFQ as the most appropriate instrument for QoL assessment in HF patients, because it has a simple structure and is easy to administer.13
Multidisciplinary HF clinics reduce mortality and hospitalizations and are a class I recommendation in European and American HF guidelines. QoL improvement is a major goal of HF clinics and treatment algorithms for all HF patients.14–16 PROs represent excellent tools to promote patient-centered care and a real opportunity to integrate patients’ voices in healthcare quality assessment.12
ObjectivesThe aim of this study was to validate a translated version of the original MLHFQ in a sample of Portuguese HF patients managed in a multidisciplinary HF clinic. In addition, this study aimed to identify associations of MLHFQ scores with other PROs measures.
MethodsStudy designThis single-center, prospective study enrolled two samples of HF patients. Pre-test sample was used for the MLHFQ translation and cultural adaptation to Portuguese. Validation sample was used to identify significant associations between the MLHFQ total and partial scores with five variables: (1) the New York Heart Association (NYHA) functional class,17 (2) the six-minute walking distance (6MWD),18 (3) the Borg fatigue score at the end of the six-minute walking test (6MWT),19 (4) anxiety score and (5) depression score assessed by the Hospital Anxiety and Depression Scale (HADS).20
A full license agreement from the University of Minnesota was obtained for validating the MLHFQ in the Portuguese language. The study adhered to the recommendations of the Declaration of Helsinki and the study protocol was approved by the local ethics committee and institutional review boards, reference number 2017.040 (040-DEFI/040-CES).
Translation and cultural adaptationThe translation process followed the steps described by Boateng et al.21 The translation into Portuguese was performed by two nonmedical independent professionals, both Portuguese native speakers and fluent English speakers. Both were aware of the aim of the study and were asked to prioritize conceptual translation. The aim of translation was to obtain a version that maintained the integrity of the instrument, preserving the meaning of the items between the two languages. To verify the content, clarity and understanding of the translation, a panel of experts composed of two cardiologists and a psychiatrist analyzed the first version. The review of equivalences included the following domains: conceptual, equivalence between items, and semantics. Changes were made when considered relevant for the cultural adaptation of the instrument, resulting in a consensus version (version 1.0). Version 1.0 was then translated into English by a native English speaker who was fluent in Portuguese, had no training in healthcare, was unaware of the original version and had not participated in the previous stages of the study. The grammatical and semantic discrepancies related to the original scale were presented to a panel of experts, and a revised version (version 2.0) was assembled. Version 2.0 was then applied to the pre-test sample. The relevance of the form of administration and response in Portuguese culture, understanding of the meaning and content of the questions, and concepts presented were assessed. The final version (version 3.0; see supplementary Table S1) incorporated all these issues.
Study samplesPre-test sample included 13 patients consecutively referred to an outpatient cardiac rehabilitation program, diagnosed with HF in accordance with the criteria of the ESC guidelines.22 Eligible patients were 18 years or older, clinically stable for the last 3 months, with no comorbidities that could impose physical limitations or inability to express themselves. All patients signed an informed consent before study participation. The MLHFQ was administered at the baseline assessment of each patient.
The validation sample included patients managed in a multidisciplinary HF clinic between February 29, 2016, and February 29, 2020, before the first coronavirus disease (COVID-19) patient was diagnosed in Portugal, in March 2020. To be admitted to the HF clinic patients had to be 18 years of age or older and either were recently hospitalized for HF, had a history of previous HF hospitalization or had frequent urgent HF visits. In the study period, a total of 321 patients were referred to the HF clinic but only 294 were eligible to MLHFQ administration. The remaining patients had at least one of the following exclusion criteria: functional dependence, unable to express themselves or to understand written questionnaires. The MLHFQ was prospectively administrated, in average 4 months after HF clinic admission, to prevent that the answers to the questionnaire reported to the period of a HF hospitalization. HF diagnosis and classification followed the Universal definition and classification of the HF consensus statement.23 The follow-up and management program was based on an outpatient ambulatory setting.24,25 The NYHA functional class was evaluated at every visit and HADS and 6MWT were regularly performed, every six to twelve months as part of the program. Only the evaluations of NYHA functional class, HADS and 6MWT performed at the same date as when the MLHFQ was administered were considered to assess the correlation between them and the MLHFQ scores. The total score of MLHFQ and results of the other tests were immediately available to the health care professionals. The following data were collected retrospectively from clinical records, at the time of HF clinic admission: age, sex, HF etiology, comorbidities, N-terminal-pro-B type natriuretic peptide (NT-proBNP) levels, estimated glomerular filtration rate (eGFR) according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation, and left ventricle ejection fraction (LVEF) values.
QuestionnairesThe MLHFQ consists of 21 questions specific to HF and appraises QoL over the previous month. Questions are answered using a Likert scale ranging from 0 to 5; 0 indicates that the question has no impact on the patient or is not applicable, and 5 indicates the greatest adverse impact. The original questionnaire can be divided into three domains: the overall QoL domain (score range 0–105), the physical domain consisting of eight questions (score range 0–40), and the emotional domain consisting of five questions (score range 0–25). A higher score represents a poorer QoL.26
Borg is a scale for rating perceived exertion and uses a Likert scale ranging from 0 to 10, which links verbal expressions to a specific score; 0 indicates “nothing at all”, and 10 indicates “very, very strong”.19
The HADS is a 14-item scale, in which all items are rated on a 4-point Likert scale, and scores are computed by summing the scores of each subscale, for anxiety or depression. Scores range from 0 to 21, where higher scores indicate more anxiety or depression symptoms.20
Statistical analysisThe categorical variables were presented as frequencies and percentages. The normality of the data was assessed through visual graphic verification and the Shapiro–Wilk test. Nonskewed continuous variables were described using means and standard deviations (SD), and variables with skewed distributions were described using medians and the respective interquartile ranges.
A significance level of 5% was assumed. Statistical analysis was performed using software R version 4.3.0. The detailed statistical methods are reported in the supplementary file.
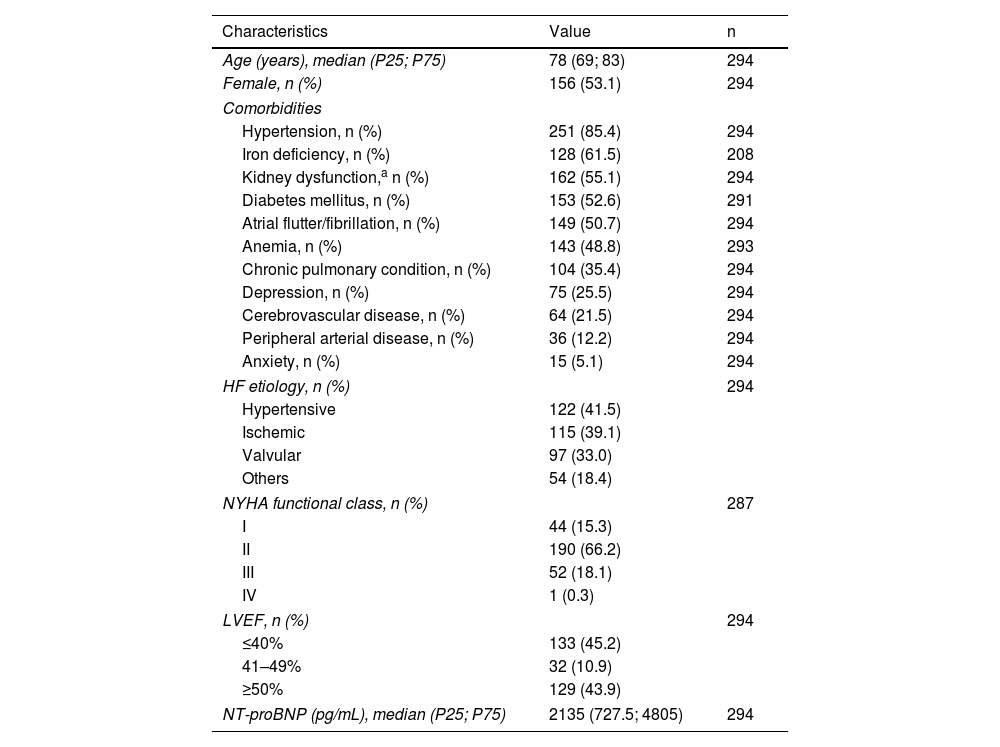
ResultsValidation sampleThe validation sample enrolled 294 patients whose baseline characteristics at HF clinic admission included the following: median age of 78 years, 53.1% female sex, a high number of comorbidities, namely, hypertension, iron deficiency, kidney dysfunction and diabetes, which were present in 85.4%, 61.5%, 55.1% and 52.6% of them, respectively. Depression and/or anxiety were present in 25.5% and 5.1% of the patients, respectively. Hypertensive heart disease was the main HF etiology (41.5%) and HF with reduced (≤40%) and preserved (≥50%) LVEF were almost equally represented (45.2% and 43.9%, respectively). Most patients were in NYHA functional class II, and the median NT-proBNP level was 2135 pg/mL (Table 1).
Baseline characteristics of the validation sample.
| Characteristics | Value | n |
|---|---|---|
| Age (years), median (P25; P75) | 78 (69; 83) | 294 |
| Female, n (%) | 156 (53.1) | 294 |
| Comorbidities | ||
| Hypertension, n (%) | 251 (85.4) | 294 |
| Iron deficiency, n (%) | 128 (61.5) | 208 |
| Kidney dysfunction,a n (%) | 162 (55.1) | 294 |
| Diabetes mellitus, n (%) | 153 (52.6) | 291 |
| Atrial flutter/fibrillation, n (%) | 149 (50.7) | 294 |
| Anemia, n (%) | 143 (48.8) | 293 |
| Chronic pulmonary condition, n (%) | 104 (35.4) | 294 |
| Depression, n (%) | 75 (25.5) | 294 |
| Cerebrovascular disease, n (%) | 64 (21.5) | 294 |
| Peripheral arterial disease, n (%) | 36 (12.2) | 294 |
| Anxiety, n (%) | 15 (5.1) | 294 |
| HF etiology, n (%) | 294 | |
| Hypertensive | 122 (41.5) | |
| Ischemic | 115 (39.1) | |
| Valvular | 97 (33.0) | |
| Others | 54 (18.4) | |
| NYHA functional class, n (%) | 287 | |
| I | 44 (15.3) | |
| II | 190 (66.2) | |
| III | 52 (18.1) | |
| IV | 1 (0.3) | |
| LVEF, n (%) | 294 | |
| ≤40% | 133 (45.2) | |
| 41–49% | 32 (10.9) | |
| ≥50% | 129 (43.9) | |
| NT-proBNP (pg/mL), median (P25; P75) | 2135 (727.5; 4805) | 294 |
HF: heart failure; LVEF: left ventricle ejection fraction; NT-proBNP: N-terminal-pro-B type natriuretic peptide; NYHA: New York Heart Association.
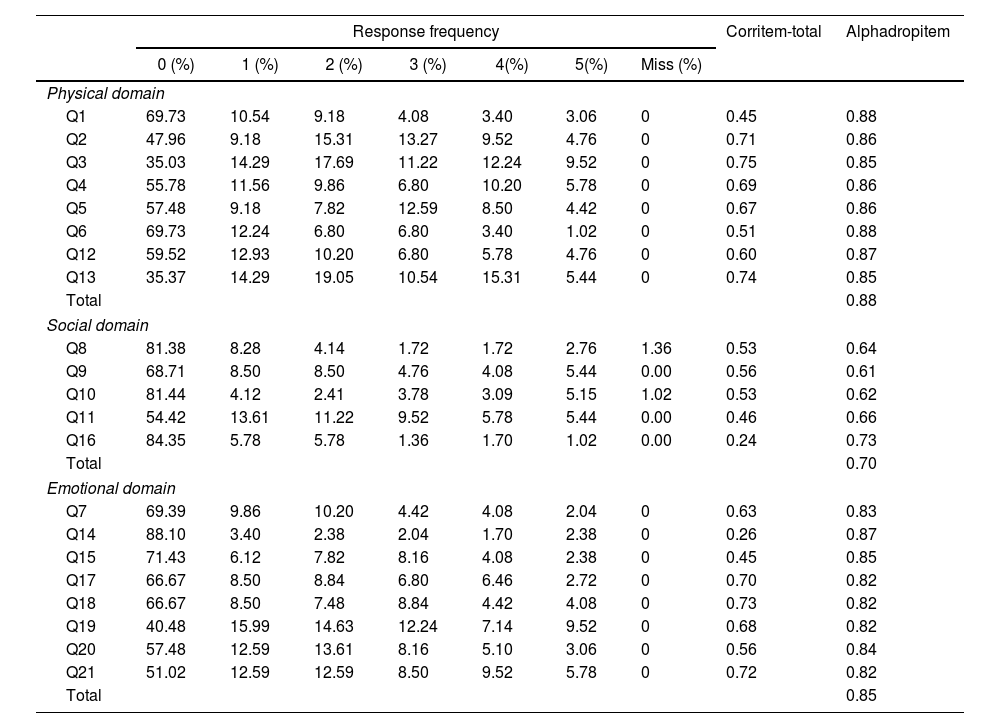
The percentage of missing values per item from the MLHFQ ranged from 0% to 1.36% (item 8). The most frequent response was “No/0”, ranging from 35.03% for item 3 to 88.10% for item 14 (Table 2).
Response frequency, item statistics and reliability.
| Response frequency | Corritem-total | Alphadropitem | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 (%) | 1 (%) | 2 (%) | 3 (%) | 4(%) | 5(%) | Miss (%) | |||
| Physical domain | |||||||||
| Q1 | 69.73 | 10.54 | 9.18 | 4.08 | 3.40 | 3.06 | 0 | 0.45 | 0.88 |
| Q2 | 47.96 | 9.18 | 15.31 | 13.27 | 9.52 | 4.76 | 0 | 0.71 | 0.86 |
| Q3 | 35.03 | 14.29 | 17.69 | 11.22 | 12.24 | 9.52 | 0 | 0.75 | 0.85 |
| Q4 | 55.78 | 11.56 | 9.86 | 6.80 | 10.20 | 5.78 | 0 | 0.69 | 0.86 |
| Q5 | 57.48 | 9.18 | 7.82 | 12.59 | 8.50 | 4.42 | 0 | 0.67 | 0.86 |
| Q6 | 69.73 | 12.24 | 6.80 | 6.80 | 3.40 | 1.02 | 0 | 0.51 | 0.88 |
| Q12 | 59.52 | 12.93 | 10.20 | 6.80 | 5.78 | 4.76 | 0 | 0.60 | 0.87 |
| Q13 | 35.37 | 14.29 | 19.05 | 10.54 | 15.31 | 5.44 | 0 | 0.74 | 0.85 |
| Total | 0.88 | ||||||||
| Social domain | |||||||||
| Q8 | 81.38 | 8.28 | 4.14 | 1.72 | 1.72 | 2.76 | 1.36 | 0.53 | 0.64 |
| Q9 | 68.71 | 8.50 | 8.50 | 4.76 | 4.08 | 5.44 | 0.00 | 0.56 | 0.61 |
| Q10 | 81.44 | 4.12 | 2.41 | 3.78 | 3.09 | 5.15 | 1.02 | 0.53 | 0.62 |
| Q11 | 54.42 | 13.61 | 11.22 | 9.52 | 5.78 | 5.44 | 0.00 | 0.46 | 0.66 |
| Q16 | 84.35 | 5.78 | 5.78 | 1.36 | 1.70 | 1.02 | 0.00 | 0.24 | 0.73 |
| Total | 0.70 | ||||||||
| Emotional domain | |||||||||
| Q7 | 69.39 | 9.86 | 10.20 | 4.42 | 4.08 | 2.04 | 0 | 0.63 | 0.83 |
| Q14 | 88.10 | 3.40 | 2.38 | 2.04 | 1.70 | 2.38 | 0 | 0.26 | 0.87 |
| Q15 | 71.43 | 6.12 | 7.82 | 8.16 | 4.08 | 2.38 | 0 | 0.45 | 0.85 |
| Q17 | 66.67 | 8.50 | 8.84 | 6.80 | 6.46 | 2.72 | 0 | 0.70 | 0.82 |
| Q18 | 66.67 | 8.50 | 7.48 | 8.84 | 4.42 | 4.08 | 0 | 0.73 | 0.82 |
| Q19 | 40.48 | 15.99 | 14.63 | 12.24 | 7.14 | 9.52 | 0 | 0.68 | 0.82 |
| Q20 | 57.48 | 12.59 | 13.61 | 8.16 | 5.10 | 3.06 | 0 | 0.56 | 0.84 |
| Q21 | 51.02 | 12.59 | 12.59 | 8.50 | 9.52 | 5.78 | 0 | 0.72 | 0.82 |
| Total | 0.85 | ||||||||
Q: question.
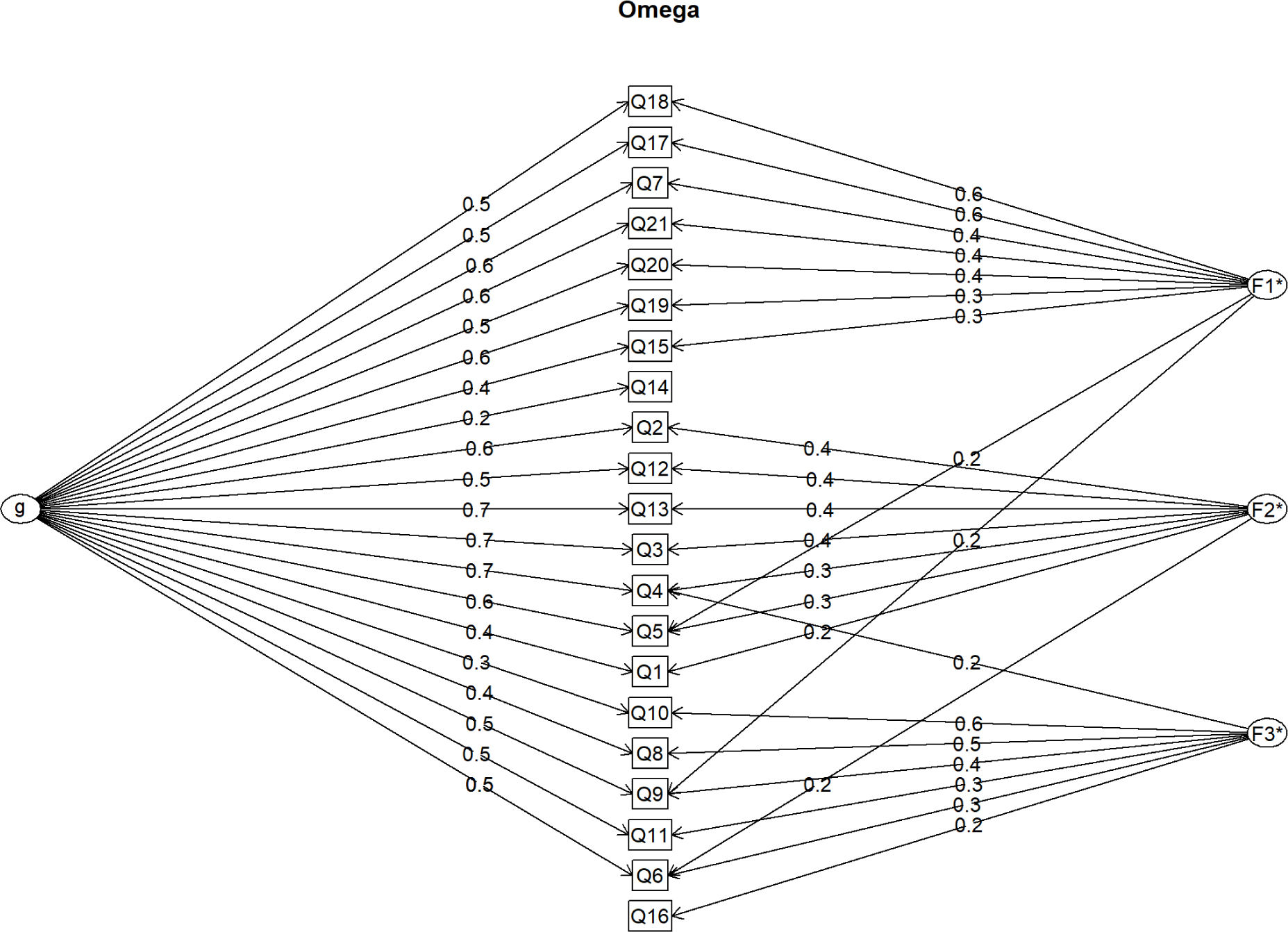
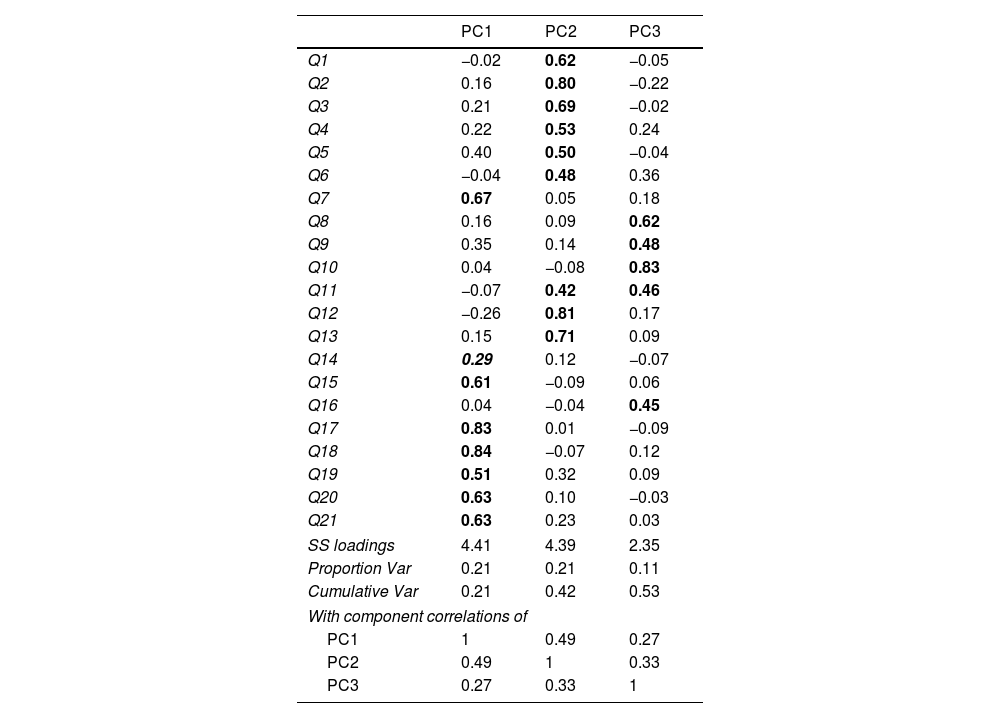
The visual inspection of the scree plot values from the principal component analysis identified three components as the final solution (Table 3). The second component included items (factor loadings higher than 0.4) related to the physical domain: 1, 2, 3, 4, 5, 6, 12 and 13. The first component included items related to the emotional domain: 7, 15, 17, 18, 19, 20 and 21. The third component included items related to the social domain: 8, 9, 10, 11 and 16. The only item with moderated standardized factor loading was item 14, which showed a correlation of 0.29 with component 1. All three components had moderate correlations with each other ranging from 0.27 to 0.49. This last result suggests the existence of a general factor.
Standardized factor loadings from principal component analysis with three components and oblimin rotation.
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Q1 | −0.02 | 0.62 | −0.05 |
| Q2 | 0.16 | 0.80 | −0.22 |
| Q3 | 0.21 | 0.69 | −0.02 |
| Q4 | 0.22 | 0.53 | 0.24 |
| Q5 | 0.40 | 0.50 | −0.04 |
| Q6 | −0.04 | 0.48 | 0.36 |
| Q7 | 0.67 | 0.05 | 0.18 |
| Q8 | 0.16 | 0.09 | 0.62 |
| Q9 | 0.35 | 0.14 | 0.48 |
| Q10 | 0.04 | −0.08 | 0.83 |
| Q11 | −0.07 | 0.42 | 0.46 |
| Q12 | −0.26 | 0.81 | 0.17 |
| Q13 | 0.15 | 0.71 | 0.09 |
| Q14 | 0.29 | 0.12 | −0.07 |
| Q15 | 0.61 | −0.09 | 0.06 |
| Q16 | 0.04 | −0.04 | 0.45 |
| Q17 | 0.83 | 0.01 | −0.09 |
| Q18 | 0.84 | −0.07 | 0.12 |
| Q19 | 0.51 | 0.32 | 0.09 |
| Q20 | 0.63 | 0.10 | −0.03 |
| Q21 | 0.63 | 0.23 | 0.03 |
| SS loadings | 4.41 | 4.39 | 2.35 |
| Proportion Var | 0.21 | 0.21 | 0.11 |
| Cumulative Var | 0.21 | 0.42 | 0.53 |
| With component correlations of | |||
| PC1 | 1 | 0.49 | 0.27 |
| PC2 | 0.49 | 1 | 0.33 |
| PC3 | 0.27 | 0.33 | 1 |
Q: question.
The numbers in bold represent the statistically significant values.
The only value in italics, “0.29”, is the only one that exhibits special behaviour in the analysis.
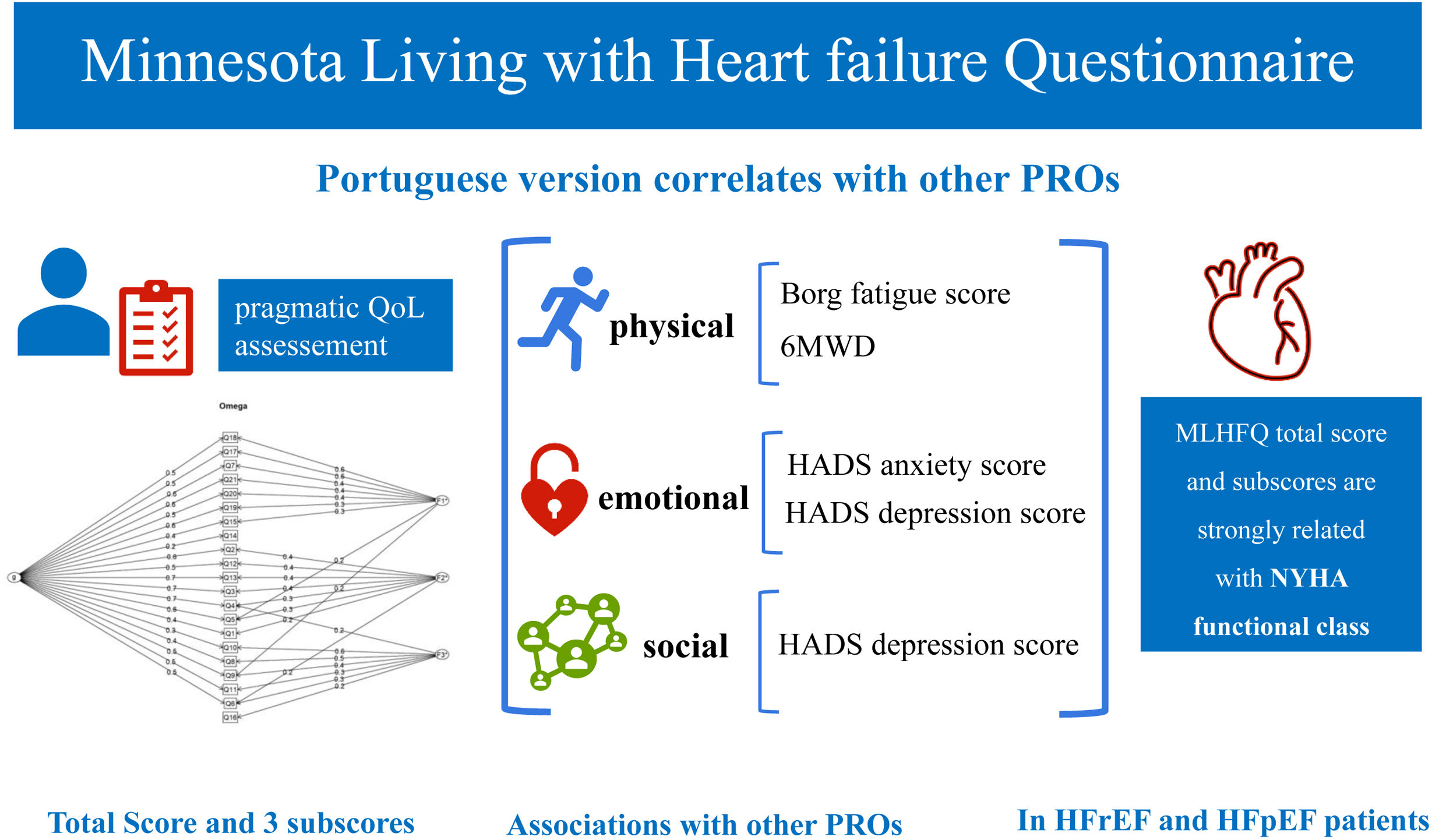
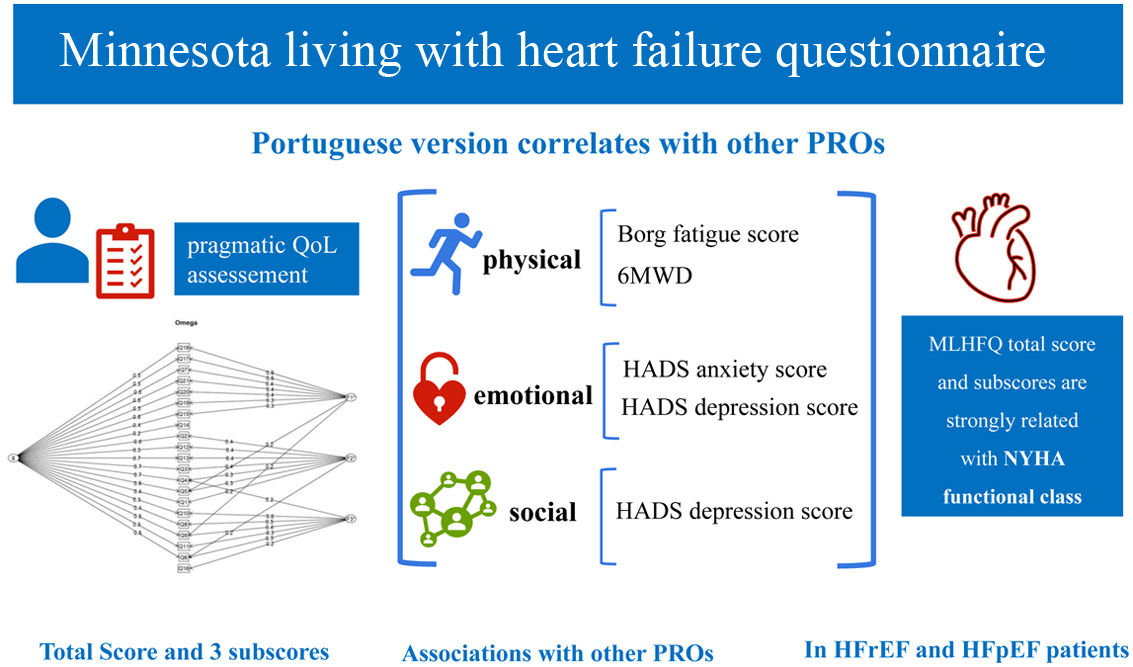
The Schmid-Leiman transformation was applied to the principal component analysis of the original dataset, and the components were rotated obliquely (Figure 1). This allowed us to identify McDonald's omega hierarchical and total as estimates of the general factor saturation of the scale. The omega hierarchical and total values were 0.70 and 0.93, respectively. Therefore, the MLHFQ has a reliable high-order factor (general factor) and good internal consistency for the scale without considering the hierarchical structure.
When the internal consistency of the three main subdomains was analyzed, the physical domain had a Cronbach's alpha of 0.88, the emotional domain had a Cronbach's alpha of 0.85, and the social domain had a Cronbach's alpha of 0.70. The internal consistency analysis revealed that the only items that slightly increased the Cronbach's alpha if dropped were items 14 and 16. Nevertheless, all the items presented item-total correlations greater than 0.4, except for items 14 and 16, which represented correlations of approximately 0.25 (Table 2).
Floor and ceiling effectsThe percentages of patients with a score of 0 (floor effect) were 6.1%, 14.2%, 20.7% and 40.4% for the general factor, physical, emotional, and social domains, respectively. The percentage of patients with the maximum score was 0% for all domains. The median scores (P25–P75) were 15 (6–33), 7 (2–15), 4.5 (1–7.4) and 2 (0–5) for general factor, physical, emotional and social domains, respectively.
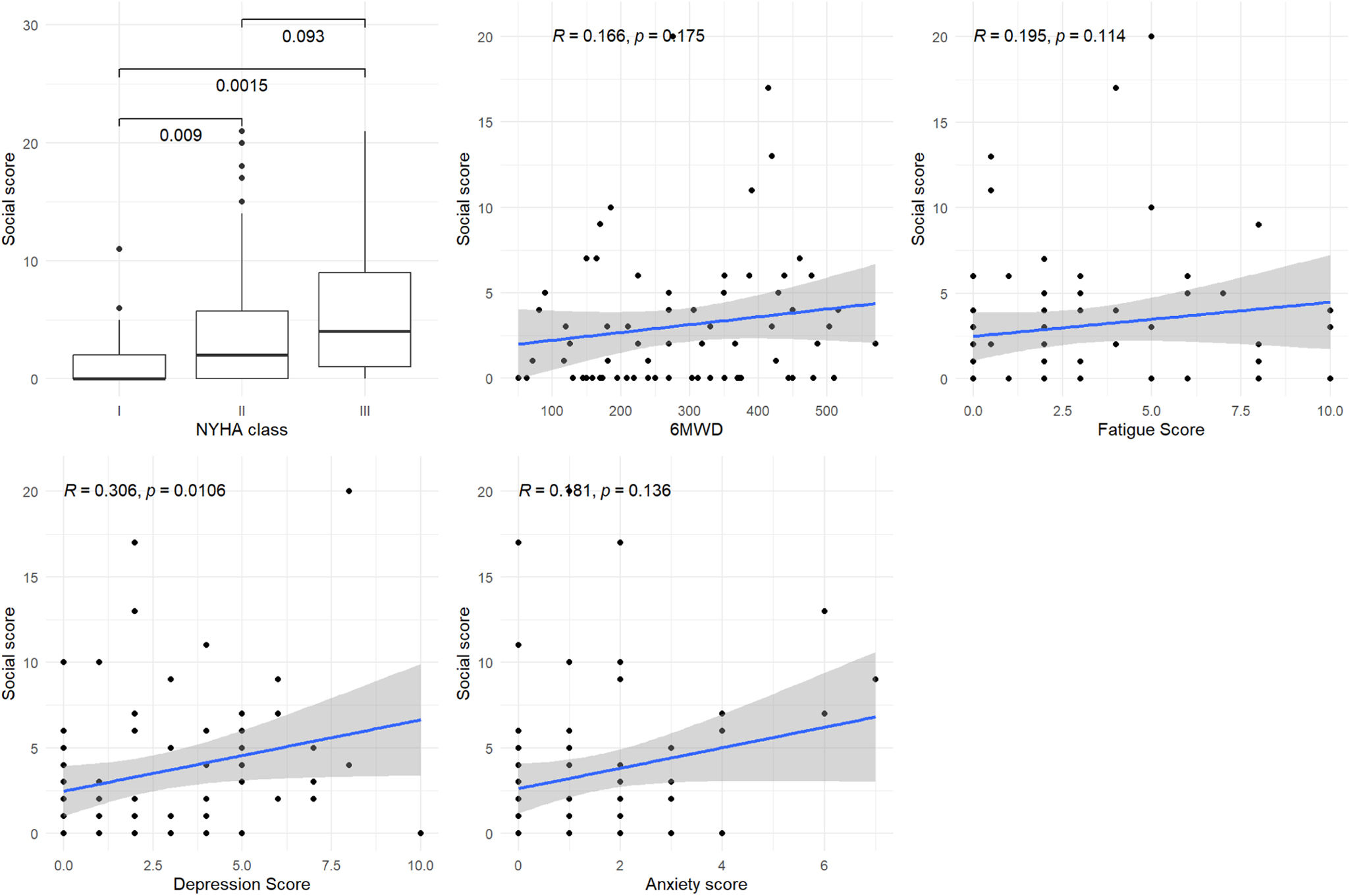
Construct validationFigures 2–5 show the associations of each domain and subdomain with the NYHA functional class, the 6MWD, the Borg fatigue score at the end of the 6MWT, and the HADS depression and anxiety scores.
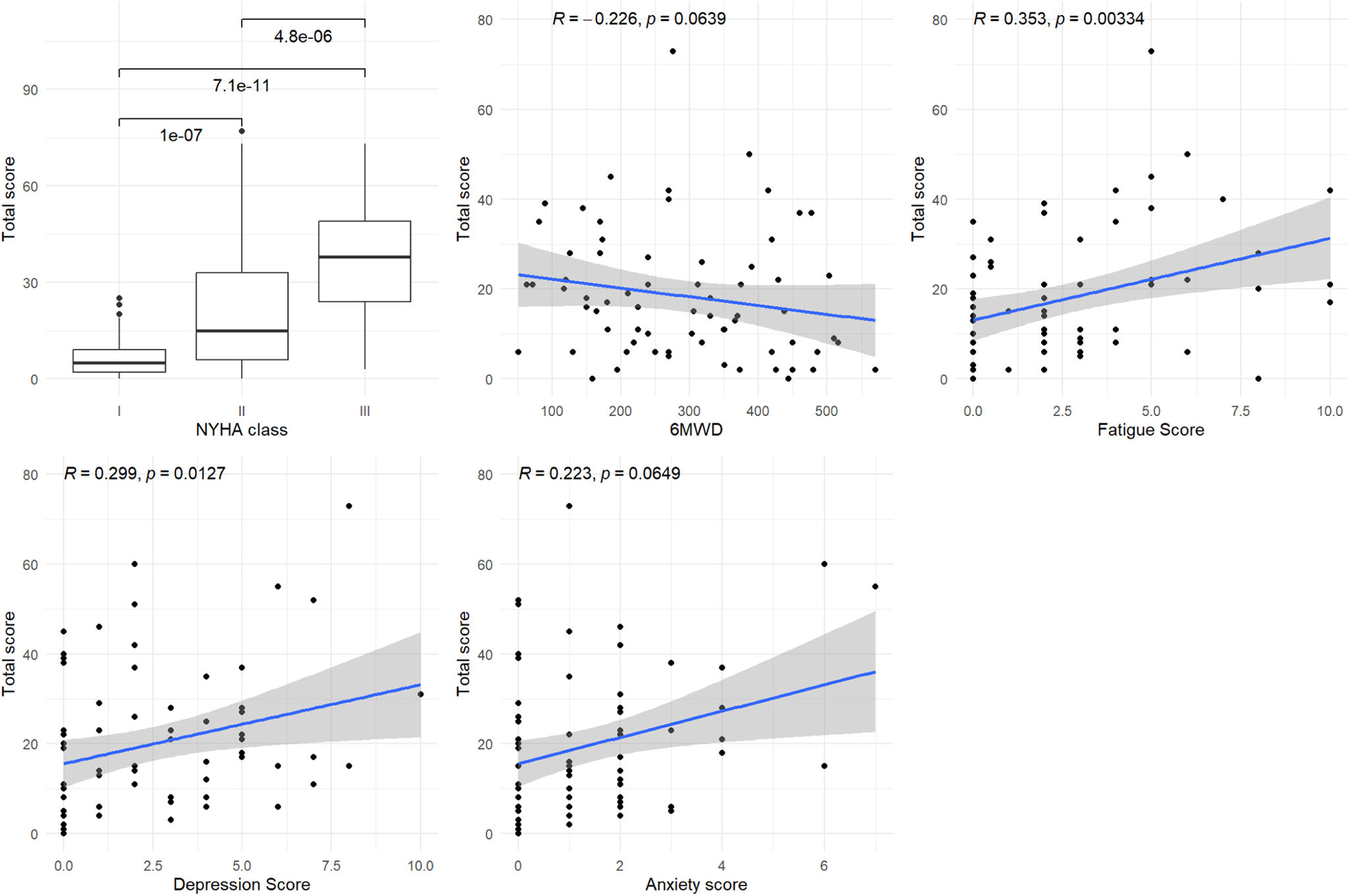
Figure 2 shows that the general factor was statistically significantly or marginally significantly associated with all the variables: NYHA functional class (I vs II and I vs III, p<0.001 and p<0.001, respectively), 6MWD (p=0.064), Borg fatigue score (p=0.003), HADS depression score (p=0.013) and HADS anxiety score (p=0.065).
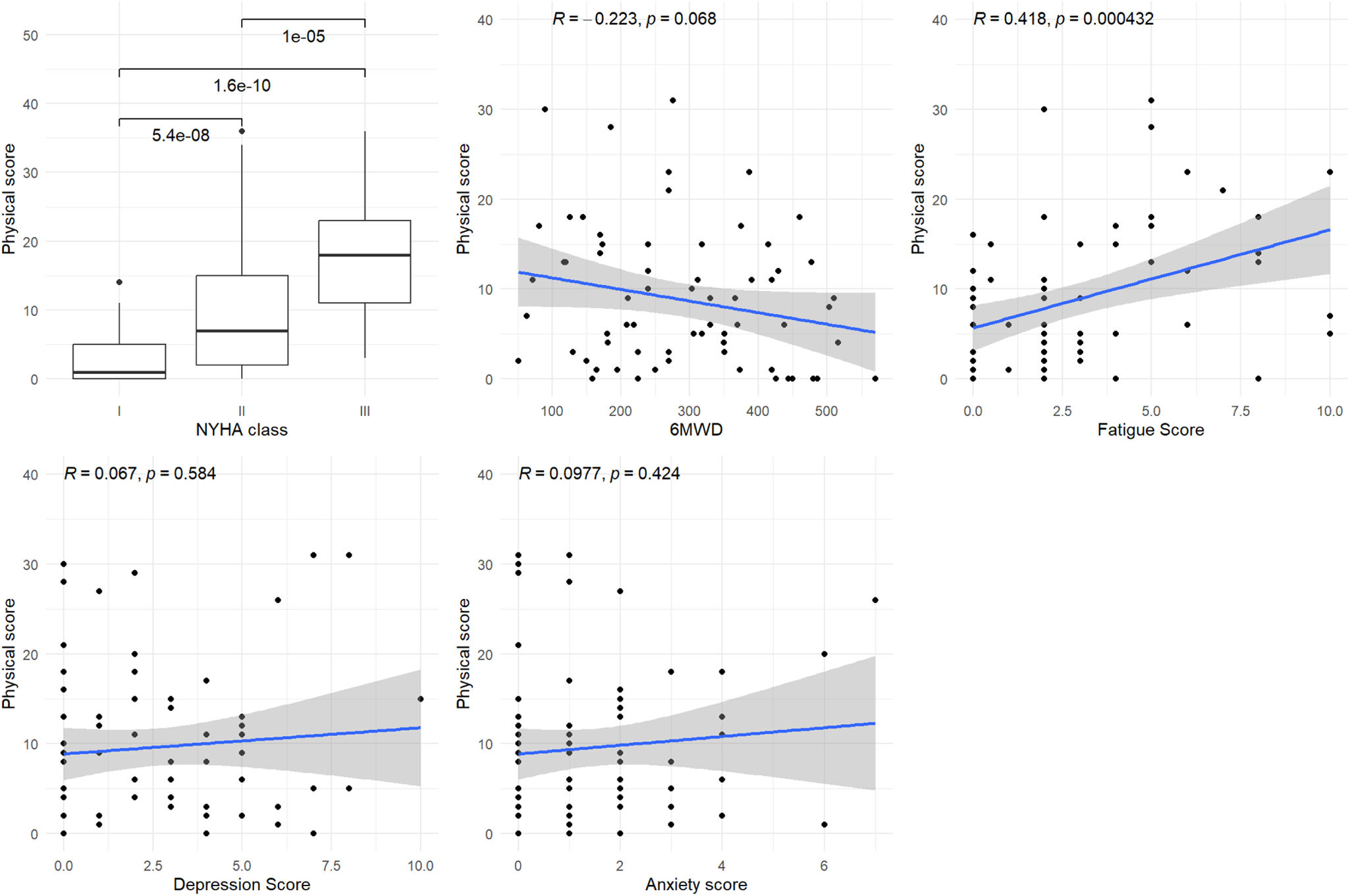
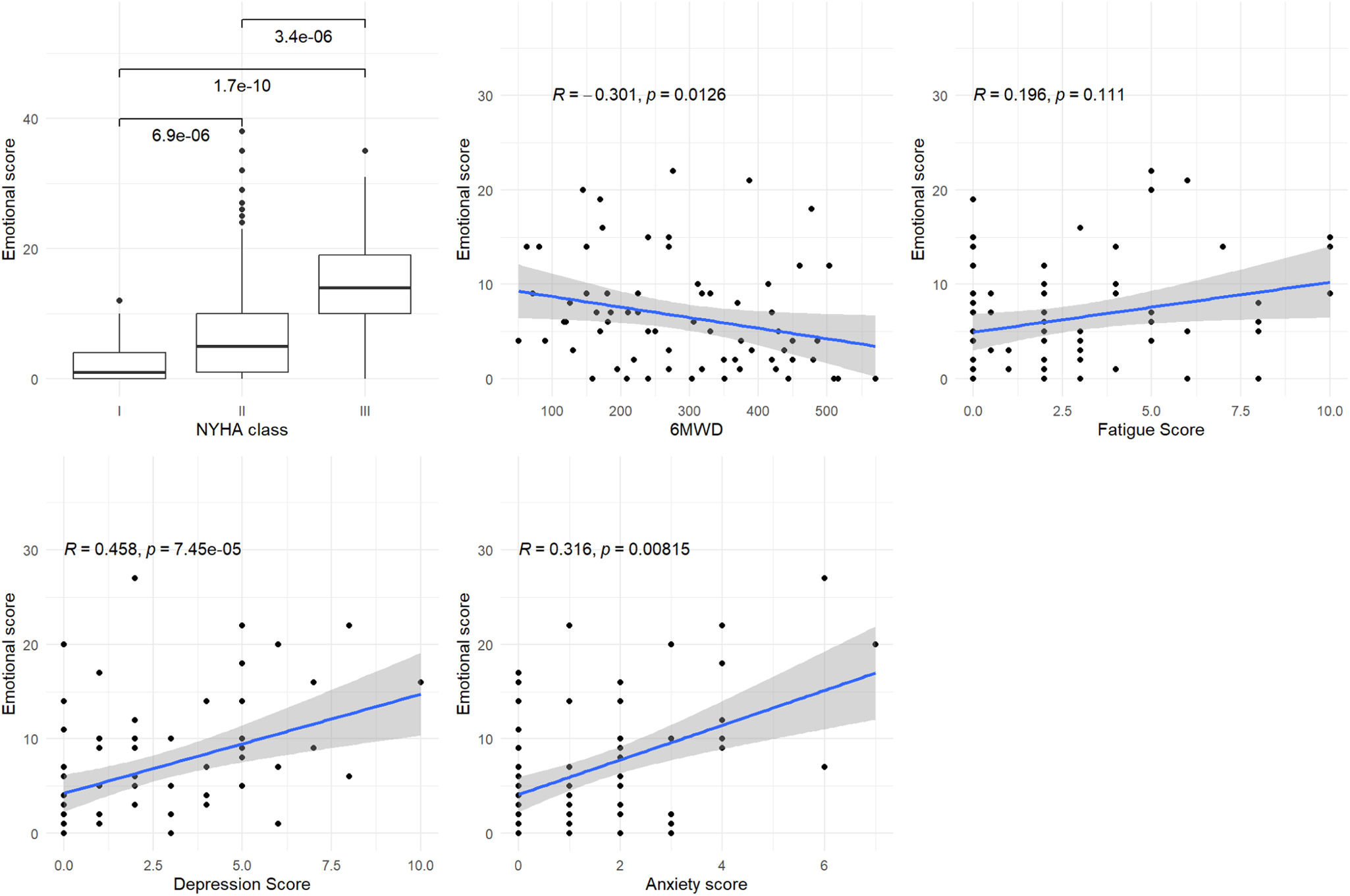
Figure 3 shows a significant or marginally significant association between the physical domain score and the 6MWD and Borg fatigue score (p=0.068 and p<0.001, respectively). Figure 4 shows a significant association between the emotional domain score and both HADS scores (p<0.001 for depression and p=0.008 for anxiety scores, respectively). Figure 5 shows a significant association between the social domain score and HADS depression score (p=0.011). Figures 2–5 demonstrate that the main domain and subdomains are strongly related to the NYHA functional class. The above findings are graphically summarized in Figure 6.
Association of MLHFQ scores with other PROs. HADS: Hospital Anxiety and Depression Scale; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; MLHFQ: Minnesota Living with Heart Failure Questionnaire; NYHA: New York Heart Association; PROs: patient-reported health outcomes; QoL: quality of life; 6MWD: six-minute walking distance.
This study aimed to validate the MLHFQ and to identify associations of MLHFQ scores with other clinical evaluations and PROs measures. Here we present the validation of the MLHFQ to European Portuguese and report the existence of three subscores: physical, emotional and social. All the scores were strongly associated with the NYHA functional class, and the MLHFQ total score was associated with all the studied variables. Additionally, to the best of our knowledge, this is the first study reporting associations between: (1) the physical score and both the Borg fatigue score and the 6MWD and (2) the emotional score and anxiety and depression HADS scores.
Recently, a working group of the ESC defined quality indicators for the care and outcomes of adults with HF, including a domain for the assessment of patients’ QoL and the recommendation of the use of a “validated tool”. The experts considered that QoL assessment by itself constitutes an accomplishment of that quality indicator, without setting any target score, given concerns about the feasibility of capturing QoL in clinical practice.2 The position of the ESC highlights the unmet need for a very pragmatic instrument in the assessment of HF-specific QoL. The MLHFQ is easy to administer, has a simple structure with 21 questions, answers displayed on a Likert scale, fits to one page only and takes approximately 5 minutes to complete.9 Gallagher et al. studied the acceptability and feasibility of implementing validated QoL instruments in HF clinics, reporting that the MLHFQ had a higher completion rate than the KCCQ, with all the questions answered by 85% vs 49% of patients, respectively.11
The validation sample in our study represented elderly patients of both sexes with many comorbidities, several HF etiologies, symptomatic HF, and similar proportion of reduced and preserved LVEF. This representation of HF patients from real world clinical practice is one of the strengths of this study. The analysis of the answers to the 21 questions of the MLHFQ revealed that there were only a few missing values. Noteworthy, 88.10% of the patients answered “No” to question 14 concerning HF hospitalization in the previous month. This may be because patients had optimized care and a low hospitalization rate.25
The Portuguese version of the MLHFQ validated in this study has a reliable total score and good internal consistency. The median total score (P25–P75) of the MLHFQ was 15 (6–33), indicating relatively good HF-related QoL, considering the range of possible total scores from 0 (zero) to 105. Gallagher et al reported an MLHFQ mean total score of 40.9±25.6 in 152 outpatients from two specialist cardiology outpatient clinics.11 It is well established that one-quarter to one-third of participants in HF trials show significant improvement in QoL when receiving placebo.27 This may reflect exposure to the beneficial environment trial, where patients are often followed more closely than in routine clinical care and healthcare providers pay particular attention to patient-reported symptoms.28 Likely, the same happens with patients under optimized care in the setting of multidisciplinary HF clinics, justifying the relatively low total score reported in this study. In fact, all the patients received education and optimized care through a HF management program, which was delivered by a multidisciplinary team of trained professionals.
This study reports the existence of three MLHFQ subscores: physical, emotional and social. This is in accordance with reports from several other authors that identified a social score.29 Bilbao et al. compared several authors’ proposals for social factors29 and recommended the use of Munyombwe et al. social factor30 because it has the best psychometric properties. When comparing the questions that compose the social score in our study and those proposed by Munyombwe et al., there is a match in questions 8, 9, 10 and 16. However, our study included question 11 but not questions 14 and 15.
Our validated version of the MLHFQ includes 20 of the 21 questions in one of the three subscores. Thus, considering subscores for the three-domain structure could be an alternative to the total score of the MLHFQ.29
The impact of HF has traditionally been measured using clinical tools, such as the NYHA classification or the 6MWT.31 The limitations of the NYHA classification include its subjective assessment and high interobserver variability, previously reported at 54%.32 In this study, the total and subdomains scores of the MLHFQ were strongly associated with the NYHA functional class. Other authors reported a significant association of HF-specific QoL scores with the NYHA functional class,11,33 highlighting the need for routine measurement of QoL in HF clinics. While QoL scores report the patient perspective, the NYHA functional class is typically rated from the clinician's perspective. Additionally, recent clinical trials have shown that the beneficial effects of HF medications in terms of symptoms, mortality and hospitalization, may not be related to improvements in QoL.28
This study revealed that the MLHFQ total score was associated with all the studied variables, namely: (1) 6MWD, (2) Borg fatigue score at the end of the 6MWT, (3) HADS depression and (4) HADS anxiety scores. These findings support the validity and reliability of the Portuguese version. The physical score was associated with fatigue and the 6MWD, the latter being an objective measure of exercise tolerance. The emotional score was significantly associated with both anxiety and depression HADS scores. However, the negative impact of anxiety and depression symptoms on the QoL of HF patients is well established in the literature.34,35
Notably, this study validated the MLHFQ in a sample with a balanced representation of HF patients with reduced and preserved LVEF. Currently, HF guidelines report interventions aimed at improving QoL only in patients with reduced LVEF.14 However, Chandra et al. reported that HF patients with preserved LVEF generally have more impaired QoL than patients with reduced LVEF. Additionally, they reported regional differences in QoL among patients with preserved LVEF.33 Thus, this study's version of the MLHFQ has potential high value, opening a door to research in the field of QoL in patients with preserved LVEF followed in Portuguese HF clinics. Nowadays, it is estimated that 650000 people live in Portugal with HF and preserved LVEF.7
Our study has some limitations that we must acknowledge. This was a single-center study conducted at an HF clinic and was not designed to include a nationally representative sample. The responsiveness of this version of the MLHFQ was not tested, although we plan to do so in the future. Nevertheless, our findings are a step forward to a better QoL assessment of Portuguese HF patients.
ConclusionsThis study supports the validity and reliability of the Portuguese MLHFQ version, recognizing the existence of a total score and three subscores: physical, emotional and social scores. All the scores were associated with the NYHA functional class. In addition, we report new and convincing evidence that the physical score is associated with the 6MWD and Borg fatigue score at the end of the 6MWT, and that the emotional score is associated with HADS anxiety and depression scores.
We believe that this MLHFQ validated version can help to overcome the challenge of QoL assessment in everyday clinical practice in Portuguese HF clinics using a pragmatic approach. Further research is needed to assess the responsiveness of the scale and the impact of routine QoL assessment in clinical practice.
CRediT authorship contribution statementIM and SV designed the study. IM, SV and AGM collected the data. IM, AGP and MS analyzed and interpreted the data. IM and MS performed the statistical analysis and prepared the manuscript. All authors vouch for the accuracy and completeness of the data and analysis. All the authors contributed to the article and read and approved the final manuscript.
Ethical approvalHealth Ethics Commission (CES) of the Porto Hospital Center. Reference number 2017.040 (040-DEFI/040-CES).
FundingThis research received no funding.
Conflicts of interestThe authors have no conflicts of interest to declare.