Among patients with aortic stenosis (AS), interstitial fibrosis has been associated with progression to heart failure and is a marker of poorer prognosis. We aimed to assess the impact of myocardial fibrosis on clinical events after aortic valve replacement (AVR) in low risk, severe AS.
MethodsWe prospectively followed 56 severe AS patients with ejection fraction >40%, who underwent AVR with simultaneous myocardial biopsies and collagen volume fraction (CVF) determination. Baseline and follow-up echocardiographic parameters were assessed. Outcomes were all-cause death and the combined endpoint of all-cause death or non-fatal cardiovascular hospitalization.
ResultsPatients were predominantly women (67.9%) and mean age was 66±12 years. At follow-up, there was a significant decrease in transaortic gradients and wall stress, as well as regression in indexed LV mass. Patients who suffered a fatal event or the combined endpoint had a higher degree of fibrosis (27.1±20.7% vs. 15.4±11.8%, p=0.035; 24.0±18.2% vs. 15.3±12.0%, p=0.038, respectively). Patients with CVF≥15.4% had higher rates of all-cause death (37.5% vs. 97.0%, p=0.001) and lower survival free of the combined endpoint of all-cause death or non-fatal cardiovascular hospitalization (0% vs. 91.2%, p<0.001). CVF was the only independent predictor of all-cause death (hazard ratio (HR) 1.88; 95% confidence interval (CI): 1.08-3.29 for each 10% increase; p=0.026) and all-cause death or cardiovascular hospitalization (HR 1.73; 95% CI: 1.03-2.911 for each 10% increase; p=0.038).
ConclusionsIn low risk AS patients, higher levels of fibrosis are independent predictors of all-cause death and the composite of all-cause death or non-fatal cardiovascular hospitalization. Further advances in anti-fibrotic therapies in AS are needed.
Na estenose aórtica, a fibrose intersticial tem sido associada a progressão para insuficiência cardíaca, sendo marcador de pior prognóstico. O nosso objetivo foi avaliar o impacto da fibrose miocárdica nos eventos clínicos após substituição valvular aórtica (SVA) em doentes com estenose aórtica (EA) grave de baixo-risco.
MétodosAvaliamos prospetivamente 56 doentes com EA grave com fração de ejeção >40% submetidos a SVA com biópsia simultânea do miocárdio e determinação da fração de volume de colagénio (FVC). Parâmetros ecocardiográficos no início e no seguimento foram avaliados. Os outcomes estudados foram mortalidade por todas as causas e o evento combinado de mortalidade por todas as causas ou hospitalização cardiovascular não fatal.
ResultadosOs doentes eram predominantemente mulheres (67,9%) e a idade média foi 66±12 anos. No follow-up, houve uma diminuição significativa do gradiente transaórtico e do stress da parede, assim como regressão da massa indexada do VE. Os doentes que sofreram um evento fatal ou o evento combinado tinham um maior grau de fibrose (27,1±20,7% versus 15,4±11,8%, p=0,035; 24,0±18,2% versus 15,3±12,0%, p=0,038, respetivamente). Os pacientes com FVC≥15,4% tiveram pior sobrevida (37,5% versus 97,0%, p=0,001) e menor sobrevivência livre do evento combinado (0% versus 91,2%, p<0,001). A FVC foi o único preditor independente de mortalidade por todas as causas (HR 1,88; 95%CI: 1,08-3,29 para um aumento de 10%; p=0,026) e mortalidade por todas as causas ou hospitalização cardiovascular (HR 1,73; 95%CI:1.03-2,911 para um aumento de 10%; p=0,038).
ConclusõesEm doentes com EA de baixo risco, níveis mais elevados de fibrose são preditores independentes de mortalidade por todas as causas e do evento composto de mortalidade por todas as causas ou hospitalização cardiovascular não fatal. São necessários avanços na terapêutica antifibrótica no contexto da EA.
In chronic pressure overload states, such as systemic hypertension (HT) and aortic stenosis (AS), the left ventricle (LV) responds with hypertrophy and altered geometry as an adaptive mechanism that helps to maintain contractile performance, despite abnormal loading conditions. Concomitantly, there are changes in cardiomyocytes and extracellular matrix connective tissue, some of them irreversible. This may help to explain the deterioration of diastolic and systolic function that take place after longstanding overload.1
Aortic valve replacement (AVR) increases long-term survival, which becomes similar to age-matched population, reduces symptoms and improves quality of life in patients with aortic valve stenosis.2,3 Late outcome after AVR depends mainly on the stage of heart disease before surgery, prosthetic-related complications, and co-morbidities.
Histopathologically, fibrosis has been implicated in the progression from a compensated phase to heart failure (HF)4 and those with higher grades of fibrosis have more severe myocardial disease and lower long-term survival rates.5,6
Our aim was to determine whether fibrosis levels in patients with severe AS but milder forms of remodeling and ejection fraction (EF) >40% had any impact on long-term clinical outcomes.
MethodsPatient selection and follow-upBetween January 2006 and December 2009, we included consecutive patients >18 years old with severe symptomatic AS (aortic valve area <1 cm2 or mean transaortic gradient ≥40 mmHg) referred for AVR at the Cardiothoracic Surgery Department of Centro Hospitalar São João, Porto, Portugal. We excluded patients with aortic regurgitation >II/IV or other significant valve diseases (>mild), significant coronary artery disease (lesions >50% on coronary angiography), EF≤40%, or previous heart surgery. All patients had to be in sinus rhythm at the time of inclusion. From the initial 141 patients included in a prospective cohort, at the time of AVR, 56 random patients underwent myocardial biopsies for collagen volume fraction evaluation and were considered for this prospective analysis. Clinical and echocardiographic follow-up was provided for all patients. Mean clinical follow-up was 5.0±2.2 years and mean final echocardiographic follow-up was 4.0±1.8 years.
The diagnosis of hypertension was performed based on the clinical records provided by the attending physician. Renal insufficiency was determined when creatinine clearance was <60 ml/kg calculated using the Cockcroft-Gaul formula.
Clinical endpoints were defined as all-cause death and a composite of all-cause death or non-fatal cardiovascular hospitalization (for HF, myocardial infarction, stroke, new-onset atrial fibrillation or advanced AV block requiring hospitalization).
Surgical techniqueAll surgery was performed using standard procedure for AVR. Patients were placed on cardiopulmonary bypass and cardiac arrest was induced and maintained with cold blood cardioplegia. The majority of patients received a bioprosthesis (64.3%) and prosthesis sizes used were <21 mm in 12.5%, 21 mm in 39.3%, 23 mm in 32.1% and 25 mm in 16.1%. At the time of surgery, patients underwent myocardial biopsies from the LV interventricular septum.
Echocardiographic studiesEchocardiographic examination was performed by a trained cardiologist and recorded on digital support. All recordings were examined by a cardiologist experienced in echocardiographic imaging at an accredited independent echocardiography laboratory (Hospital Clínico San Carlos in Madrid, Spain), blinded to patient details. Studies were performed using Phillips IE-33 equipment with a S5-1 transducer and M-mode, two-dimensional, pulsed, continuous, color-flow and tissue Doppler capabilities. Correct orientation of imaging planes, cardiac chambers dimensions and function measurements were performed according to the European Association of Echocardiography (EAE)/American Society of Echocardiography (ASE) recommendations.7
Left ventricle mass was estimated according to the joint recommendations of the ASE and EAE using Devereux's formula for ASE measurements in diastole: LV mass=0.8×(1.04×([LV internal dimension+posterior wall thickness+interventricular septal thickness]3−[LV internal dimension]3)+0.6 g.7 Left ventricular hypertrophy was defined by an LV mass index >115 g/m2 in men and >95 g/m2 in women.
Relative wall thickness (RWT) was calculated for the assessment of LV geometry using the formula 2× posterior wall thickness/LV diastolic diameter. Increased RWT was present when this ratio was >0.42.7
LA volume was measured in LV end systole in the frame preceding mitral valve opening. The volume was measured using the biplane area length method and corrected for body surface area. Aortic valve area was estimated using quantitative Doppler continuity equation.
Mitral inflow was assessed in the apical four-chamber view using pulsed wave Doppler with the sample volume placed at the tips of mitral leaflets during diastole. From the mitral inflow profile, the peak flow velocity of early filling (E wave), peak flow velocity of atrial contraction (A wave), the E/A ratio, and early filling deceleration time (DT) were measured. Doppler tissue imaging (DTI) of the mitral annulus was obtained from the apical four-chamber using a sample volume placed in the septal mitral valve annulus. The peak systolic annular velocity (Sm) and early diastolic septal velocity (e’) were determined, and the E/e’ ratio was derived.
As a measure of global LV load, we calculated the valvulo-arterial impedance: Zva=(SAP+MG)/SVI, where SAP is the systolic arterial pressure and MG is the mean transvalvular pressure gradient and SVI is stroke volume index.8
Histological determination of fibrosisLight microscopic quantification of fibrosis has previously been described and validated.9 Fibrosis analysis of myocardial biopsies was performed using picrosirius-red–stained, 4-μm-thick-sections of tissue (±5 sections of each sample). Images of these sections were acquired with a projection microscope (×50). Subsequent image analysis with Slidebook 4.0 software (3I, Denver, Colo) was performed to determine the extent of reactive interstitial fibrosis, which was expressed as collagen volume fraction (%). Areas of reparative and perivascular fibrosis were excluded. Myocardial fibrosis was calculated as the sum of all connective tissue areas divided by the sum of connective tissue and muscle areas averaged over four to six representative fields of the section in 18 male and 38 female AS patients. In our laboratory, the normal value of fibrosis for LV myocardial biopsy material is 5.4±2.2%.
Statistical analysisCategorical variables were expressed as percentages and continuous variables as mean ± standard deviation unless otherwise specified. Continuous variables were compared between groups using an unpaired t-test (for normally distributed variables) or the Mann–Whitney U-test (for non-normally distributed variables). For comparison between baseline and follow-up, a paired Student's t-test was applied or a Wilcoxon test (for non-normally distributed variables). Chi-square test (or Fisher exact test) was used to compare categorical variables. Univariable binary logistic regression models (Wald method, p=0.05/0.20 for covariate inclusion/exclusion) were used in conjunction with the area under the curve (ROC) to assess the best cutoff-point (highest sensitivity and specificity) of a continuous variable to predict a particular outcome of all-cause death and all-cause death and cardiovascular hospitalization. The Kaplan-Meier and Cox models were used to assess survival times after surgery for all-cause death, for non-fatal cardiovascular hospitalization, and for the composite of all-cause death or cardiovascular hospitalization, and the log-rank test was used to compare survival curves. All reported probability values are two-tailed, and p<0.05 was considered statistically significant. Analyses were performed with the IBM® SPSS® Statistics software package (version 22.0) (SPSS Inc, Chicago, IL, USA).
ResultsPatients were mainly women (67.9%) and mean age was 66.3±11.5 years (Table 1). They had severe AS (aortic valve area of 0.41±0.13 cm2), the majority were mildly symptomatic (New York Health Association (NYHA) class II at 76.8%), had low risk of operative mortality (mean Euroscore II of 1.5±1.0%), and global systolic function was normal or mildly compromised, with a mean LVEF of 63.7±7.6% (three patients had EF<50% and the minimum was 42.7%).
Clinical characteristics of aortic stenosis patients.
| n=56 | |
|---|---|
| Gender (Female) [n (%)] | 38 (67.9%) |
| Age | 66.3±11.5 |
| BSA | 1.7±0.2 |
| Euroscore II | 1.5±1.0 |
| HT [n (%)] | 27 (48.2%) |
| DM [n (%)] | 10 (17.9%) |
| CKD [n (%)] | 22 (39.3%) |
| GFR (ml/min) | 66.2±20.8 |
| NYHA≥3 [n (%)] | 13 (23.2%) |
| LVH basal [n (%)] | 47 (83.9%) |
| LVH final [n (%)] | 24 (47.1%) |
BSA: body surface area; CKD: chronic kidney disease; DM: diabetes mellitus; GFR: glomerular filtration rate; HT: hypertension; LVH: left ventricle hypertrophy; NYHA: functional class of New York Heart Association. Results are presented as means ± standard deviation unless otherwise noted.
In the last echocardiographic evaluation, performed 4.0±1.8 years after surgery, patients experienced a significant decrease in transaortic gradients and wall stress, as well as regression in indexed LV mass (LVMI) (Table 2). The median absolute and relative decrease in LVMI was 20.9 g (p25-75: 1.0-39.9 g) and 17.2% (p25-75: 1.0-26.9%), respectively.
Baseline and final echocardiographic characterization of aortic stenosis patients.
| Basal | Final | p | |
|---|---|---|---|
| LV geometry | |||
| Interventricular septum (cm) | 1.45±0.26 | 1.27±0.23 | <0.001 |
| Posterior wall (cm) | 1.08±0.18 | 1.02±0.17 | 0.033 |
| Relative wall thickness | 0.47±0.10 | 0.45±0.09 | 0.126 |
| LV mass index (g/m2) | 129.5±31.9 | 109.8±30.8 | 0.000 |
| LV end-systolic volume index (ml/m2) | 17.8±7.0 | 18.5±8.8 | 0.779 |
| LV end-diastolic volume index (ml/m2) | 48.2±12.5 | 49.4±17.2 | 0.819 |
| Aortic/Prosthesis stenosis severity | |||
| Maximal transaortic velocity (cm/s) | 472.4±69.8 | 263.0±73.6 | <0.001 |
| Maximal transaortic gradient (mmHg) | 89.6±26.2 | 29.4±17.6 | <0.001 |
| Medium transaortic gradient (mmHg) | 54.7±16.5 | 16.1±8.7 | <0.001 |
| Effective orifice area index (cm/m2) | 0.41±0.13 | 0.86±0.27 | <0.001 |
| Increase effective orifice area (%) | 124.5±83.3 | ||
| Hemodynamic load | |||
| Valvulo-arterial impedance (mmHg/ml/m2) | 6.6±2.6 | 5.8±2.3 | 0.072 |
| Peak LV wall stress (dynes/cm2) | 227.1±89.6 | 168.3±41.6 | 0.005 |
| Systolic function | |||
| Ejection fraction (%) | 63.7±7.6 | 64.3±6.9 | 0.525 |
| Systolic annular velocity (cm/s) | 5.53±1.11 | 6.01±1.10 | 0.159 |
LV: left ventricle.
Values are means±SD unless otherwise indicated.
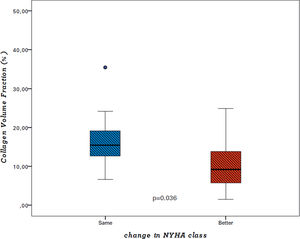
Median value of collagen volume fraction (CVF), assessed at the time of surgery, was 12.8% (p25-75: 7.7-18.8%). There was no correlation between CVF and preoperative LVMI, LV diameters or volumes, relative wall thickness or EF. Although patients in NYHA class ≥III before surgery had higher levels of myocardial fibrosis, this difference was not statistically significant (CVF 21.2±15.8% vs. 15.6±12.7%, p=0.327). When comparing final with baseline NYHA class, patients with functional improvement after AVR had lower CVF values at the time of surgery (11.5±9.3% vs. 17.3±8.4%, p=0.036) (Figure 1).
Collagen volume fraction was higher in patients with persistent left ventricular hypertrophy (LVH) late after aortic valve replacement (20.0±14.6% vs. 13.2±11.5%, p=0.027).
Clinical outcomesAt the end of 5.0±2.2 years of follow-up, there were seven deaths (12.5%) and four non-fatal cardiovascular hospitalizations (7.1%, two patients for HF, one for de novo atrial fibrillation and one for biologic prosthesis dysfunction).
In patients with an event (all-cause death or cardiovascular hospitalization) there were no significant differences in baseline clinical characteristics (Table 3) compared with those who did note experience events. On the preoperative echocardiogram, these patients had worse longitudinal function, with a lower peak systolic annular velocity (Sm) (Table 3), and no differences in AS severity, LV mass or EF.
Baseline characterization according to the occurence of all-cause death or non-fatal cardiovascular hospitalization.
| All-cause death or non-fatal cardiovascular hospitalization | |||
|---|---|---|---|
| No (n=46) | Yes (n=10) | p | |
| Sex (Female) [n (%)] | 30 (65.2%) | 8 (80.0%) | 0.474 |
| Age | 64.7±11.6 | 73.8±8.0 | 0.260 |
| Euroscore II | 1.58±0.74 | 1.15±0.18 | 0.414 |
| HT [n (%)] | 21 (45.7%) | 6 (60.0%) | 0.497 |
| DM [n (%)] | 8 (17.4%) | 2 (20.0%) | 1.000 |
| CKD [n (%)] | 17 (37.0%) | 5 (50.0%) | 0.490 |
| GFR (ml/min) | 59.0±16.8 | 79.4±30.3 | 0.293 |
| NYHA≥3 [n (%)] | 10 (21.7%) | 3 (30.0%) | 0.682 |
| LVH basal [n (%)] | 38 (82.6%) | 9 (90.0%) | 1.000 |
| LVH final [n (%)] | 17 (37.0%) | 7 (70.0%) | 0.042 |
| LV geometry | |||
| LV end-diastolic diameter index (cm/m2) | 2.66±0.38 | 2.65±0.29 | 0.563 |
| LV end-sistolic diameter index (cm/m2) | 1.76±0.28 | 1.63±0.12 | 0.078 |
| Relative wall thickness | 0.46±0.10 | 0.43±0.01 | 0.422 |
| LV mass index (g/m2) | 120.6±34.5 | 119.1±8.5 | 0.684 |
| LV end-systolic volume index (ml/m2) | 48.7±12.2 | 26.6±5.2 | 0.147 |
| LV end-diastolic volume index (ml/m2) | 19.2±6.7 | 8.4±1.0 | 0.095 |
| Aortic/Prosthesis stenosis severity | |||
| Maximal transaortic velocity (cm/s) | 456.4±58.9 | 500.1±85.8 | 0.283 |
| Effective orifice area index (cm/m2) | 0.38±0.11 | 0.41±0.05 | 0.974 |
| Hemodynamic load | |||
| Valvuloarterial impedance (mmHg/ml/m2) | 6.7±1.6 | 10.1±3.5 | 0.404 |
| Peak LV wall stress (dynes/cm2) | 227.3±51.7 | 241.1±52.4 | 0.699 |
| Diastolic function | |||
| E/A | 0.77±0.26 | 1.03±0.64 | 0.181 |
| e’ | 5.2±1.7 | 5.1±1.9 | 0.836 |
| E/e’ | 17.6±6.3 | 21.8±0.35 | 0.240 |
| Systolic funciton | |||
| Ejection fraction (%) | 61.0±7.9 | 68.3±2.3 | 0.330 |
| Systolic annular velocity (cm/s) | 5.37±1.13 | 4.38±0.56 | 0.031 |
| Collagen volume fraction (%) | 24.0±18.2 | 15.3±12.0 | 0.038 |
CKD: chronic kidney disease; DM: diabetes mellitus; GFR: glomerular filtration rate; HT: hypertension; LV: left ventricle; LVH: left ventricle hypertrophy; NYHA: functional class of New York Heart Association. Results are presented as means ± standard deviation unless otherwise noted.
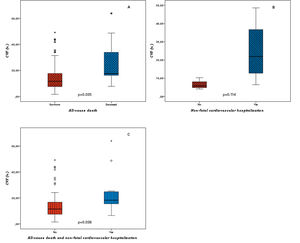
There was a positive association between the level of fibrosis, assessed by CVF, and clinical outcomes (Figure 2). Patients who died had a significantly higher degree of fibrosis at the time of surgery (27.1±20.7% vs. 15.4±11.8%, p=0.035), and the same trend was observed for non-fatal cardiovascular hospitalization (24.8±17.7% vs. 6.7±3.2%, p=0.114). Myocardial fibrosis levels were also higher in those with the composite outcome of all-cause death or non-fatal cardiovascular hospitalization (24.0±18.2% vs. 15.3±12.0%, p=0.038).
After multivariate Cox regression analysis, CVF was the only independent predictor of all-cause death (hazard ratio (HR) 1.88; 95% confidence interval (CI): 1.08-3.29, for each 10% increase; p=0.026) and all-cause death or cardiovascular hospitalization (HR 1.73; 95%CI:1.03-2.911, for each 10% increase; p=0.038) (Table 4).
Univariate and multivariate associations with all-cause death and the outcome of all-cause death and non-fatal cardiovascular hospitalization.
| Variable | p | All-cause death | All-cause death and non-fatal cardiovascular hospitalization | |||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | p | Multivariable analysis | p | Univariable analysis | p | Variable | ||
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |||||
| Sex | 0.370 | 2.035 (0.431-9.614) | 0.306 | 3.025 (0.363-25.179) | ||||
| Age (10 y increment) | 0.022 | 2.509 (1.142-5.515) | 0.171 | 1.684 (0.798-3.552) | 0.035 | 2.802 (1.077-7.288) | ||
| HT | 0.307 | 1.938 (0.545-6.983) | 0.525 | 1.627 (0.363-7.284) | ||||
| DM | 0.754 | 1.283 (0.271-6.066) | 0.380 | 2.09 (0.403-10.844) | ||||
| Euroscore II | 0.013 | 1.553 (1.099-2.195) | 0.012 | 1.626 (1.112-2.378) | 0.059 | 1.519 (0.984-2.345) | ||
| GFR | 0.138 | 0.975 (0.944-1.008) | 0.198 | 0.973 (0.934-1.014) | ||||
| NYHA≥III | 0.639 | 1.383 (0.357-5.354) | 0.710 | 1.365 (0.265-7.046) | ||||
| LVMI | 0.992 | 1.000 (0.980-1.020) | 0.948 | 1.001 (0.977-1.025) | ||||
| EOAI | 0.682 | 0.312 (0.001-81.836) | 0.845 | 0.532 (0.001-294.369) | ||||
| SVI | 0.641 | 0.979 (0.896-1.070) | 0.583 | 0.972 (.877-1.077) | ||||
| Zva | 0.600 | 1.065 (0.841-1.350) | 0.938 | 1.013 (0.732-1.401) | ||||
| EF | 0.274 | 1.060 (0.955-1.176) | 0.641 | 1.028 (0.915-1.156) | ||||
| Residual LVH | 0.058 | 4.591 (0.952.22.138) | 0.097 | 3.825 (0.783-18.695) | 0.284 | 2.528 (0.463-13.815) | ||
| CVF (10% increments) | 0.014 | 1.681 (1.113-2.539) | 0.038 | 1.733 (1.032-2.911) | 0.013 | 1.811 (1.135-2.889) | 0.026 | 1.882 (1.078-3.289) |
Variables included in multivariable analysis: age, Euroscore II, glomerular filtration rate, residual LVH, collagen volume fraction, DM: diabetes mellitus; CVF: collagen volume fraction; EF: ejection fraction; EOAI: effective orifice area index; GFR: glomerular filtration rate (MDRD formula); HR: hazard ratio; HT: arterial hypertension; LVH: left ventricular hypertrophy at follow-up; LVMI: left ventricular mass index;
NYHA: functional class of New York Heart Association; SVI: stroke volume index; Zva: valvuloarterial impedance.
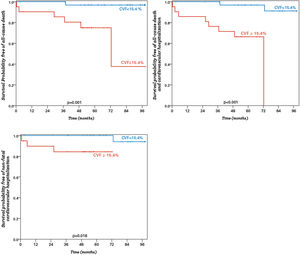
A cut-off of 15.4% for CVF was used to calculate probability of survival free of events. This value was chosen after ROC curve analysis to assess the best CVF cutoff-point to predict the outcome of all-cause death (area under curve (AUC) 0.75, p=0.036) and all-cause death and cardiovascular hospitalization (AUC 0.92, p=0.038). Comparative characteristics of patients with CVF≥15.4% vs. CVF<15.4% can be seen in Table 5 and Table 6. Patients with CVF≥15.4% had a lower probability of survival (37.5% vs. 97.0%, p=0.001), lower probability of survival free of non-fatal cardiovascular hospitalization (84.4% vs. 94.1%, p=0.018) and lower probability of survival free of the composite of all-cause death or non-fatal cardiovascular hospitalization (0% vs. 91.2%, p<0.001) (Figure 3).
Clinical characterization according to collagen volume fraction.
| CVF≥15.4% | CVF<15.4% | p | ||
|---|---|---|---|---|
| Gender (Female) | n (%) | 16 (76.2%) | 22 (62.9%) | 0.301 |
| Age (year) | 68.4±11.3 | 65.1±11.8 | 0.297 | |
| BSA | 1.7±0.2 | 1.8±0.2 | 0.799 | |
| Euroscore II (%) | 1.9±1.4 | 1.4±0.7 | 0.182 | |
| HT [n (%)] | n (%) | 11 (52.4%) | 16 (45.7%) | 0.629 |
| DM [n (%)] | n (%) | 6 (28.6%) | 4 (11.4%) | 0.152 |
| CKD [n (%)] | n (%) | 10 (47.6%) | 12 (34.3%) | 0.323 |
| GFR (ml/min) | 61.2±20.7 | 69.3±20.6 | 0.130 | |
| NYHA ≥3 [n (%)] | n (%) | 7 (33.3%) | 6 (17.1%) | 0.201 |
| LVH basal [n (%)] | n (%) | 19 (90.5%) | 28 (80%) | 0.459 |
| LVH final [n (%)] | n (%) | 13 (72.2%) | 11 (33.3%) | 0.008 |
BSA: body surface area; CKD: chronic kidney disease; CVF: collagen volume fraction; DM: diabetes mellitus; GFR: glomerular filtration rate; HT: hypertension; LVH: left ventricle hypertrophy; NYHA: functional class of New York Heart Association. Results are presented as means ± standard deviation unless otherwise noted.
Echocardiographic characterization according to collagen volume fraction.
| CVF ≥15.4% | p | CVF <15.4% | p | |||
|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Preoperative | Postoperative | |||
| Relative wall thickness | 0.46±0.9 | 0.48±0.11 | 0.525 | 0.48±0.11 | 0.43±0.1 | 0.015 |
| LV mass index (g/m2) | 133.3±35.3 | 114.1±20.4 | 0.023 | 127.4±30.3 | 107.5±35.3 | 0.002 |
| LV end-systolic volume index (ml/m2) | 45.8±9.7 | 46.5±16.3 | 0.893 | 49.6±13.8 | 51.2±17.6 | 0.674 |
| LV end-diastolic volume index (ml/m2) | 40.2±13.0 | 41.8±9.6 | 0.652 | 31.3±13.3 | 39.7±11.0 | 0.680 |
| Maximal transaortic velocity (cm/s) | 4.83±0.82 | 2.63±0.69 | <0.001 | 4.68±0.67 | 2.63±0.76 | <0.001 |
| Effective orifice area index (cm/m2) | 0.39±0.11 | 0.95±0.34 | <0.001 | 0.42±0.14 | 0.81±0.21 | <0.001 |
| Peak systolic anular velocity (cm/s) | 5.7±0.6 | 5.5±1.3 | 0.484 | 5.5±1.2 | 6.2±1.0 | 0.065 |
| E’(cm/s) | 4.8±1.3 | 5.7±2.1 | 0.145 | 5.4±2.1 | 5.7±1.8 | 0.441 |
| E/e’ | 18.9±5.8 | 19.2±7.3 | 0.882 | 15.5±5.3 | 16.6±5.5 | 0.310 |
| Ejection fraction (%) | 64.1±7.7 | 62.7±7.2 | 0.515 | 63.5±7.7 | 65.2±6.6 | 0.223 |
| Stroke volume index (ml/m2) | 29.6±8.2 | 30.2±10.3 | 0.876 | 31.4±3.3 | 33.0±9.9 | 0.527 |
CVF: collagen volume fraction; LV: left ventricle.
Results are presented as mean ± standard deviation.
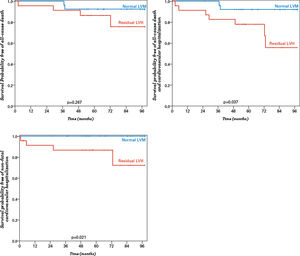
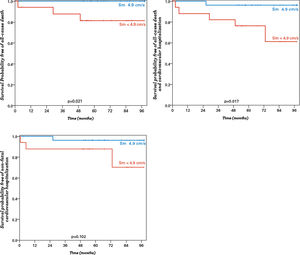
Patients with residual LVH after surgery had a lower survival free of a combined event (all-cause death or non-fatal cardiovascular hospitalization), when compared with those with normal LVM (70.8% vs 92.6%, p=0.037) (Figure 4). Moreover, patients with Sm of <4.9 cm/s had significantly worse survival (81.6% vs. 100.0%, p=0.021) and worse survival free from death or non-fatal cardiovascular hospitalization (55.8% vs. 96.3%, p=0.017; AUC 0.77, p=0.027) (Figure 5).
We found that in our low risk cohort of patients with severe AS, higher levels of fibrosis have a negative prognostic impact with lower survival free of all-cause death or the composite of all-cause death or non-fatal cardiovascular hospitalization. Moreover, it was a predictor of events, independent of other well established prognostic factors such as EF, age, baseline LVMI or NYHA class. For our patients, a cut-off value of 15% for CVF had a good performance as a predictor of clinical events.
Other authors have already described the prognostic importance of myocardial fibrosis in AS, either using histological assessment or noninvasive evaluation by cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE).5,6,10–12 However, most of these studies analyzed patients with worse preoperative NYHA class and more advanced forms of myocardial disease when compared with our cohort. Patients usually had lower values of EF, higher LV volumes and dimensions and higher levels of histological interstitial fibrosis, suggestive of more extensive remodeling.5,6,10 Likewise, the inclusion of aortic regurgitation patients5 or the coexistence of other cardiovascular comorbidities, such as atrial fibrillation and coronary artery diasease,10,12 which were excluded in our study, may have influenced outcomes.
Milano et al.6 performed a very similar study in a group of 99 patients with AS in whom fibrosis was calculated from myocardial biopsies obtained during surgery. In their retrospective analysis, the 10 year survival rate was lower in patients with severe fibrosis (defined as fibrous index >50%) and no significant improvement in NYHA class was seen in this the group. We also found worse long-term survival and a lower NYHA class improvement in those with more severe fibrosis, but ours was a prospective study and our cut-off point was much lower. According to Milano et al.’s criteria (no or mild fibrosis if <20% and moderate fibrosis if 20-50%), most of our patients would have been included in the group with mild fibrosis. This can help to explain why our patients with higher level of fibrosis have less ventricular remodeling and dysfunction (only 9.5% of those with CVF≥15.4% have EF<50%) compared with their group with moderate or severe fibrosis. Moreover, unsurprisingly, we did not find a significant correlation between fibrosis level and LV diameters, RWT or EF, which was described in the aforementioned work. It is expected for these correlations to be stronger in more severe grades of fibrosis. Even with less severe forms of myocardial disease, we still found an increase in events in our AS patients as the levels of fibrosis increased.
Myocardial fibrosis in aortic stenosisFibrosis is an early morphological alteration in patients with AS and has been cited as one of the reasons for impaired LVH regression after AVR.13 Once established, fibrosis is a major determinant of diastolic and systolic dysfunction and it is one of the structural substrates for arrhythmogenicity, thus playing a major role in sudden death and the progression of HF.4,14 While myocyte hypertrophy is dependent on load, fibrosis seems also to be regulated by non-hemodynamic factors, such as neurohormones.15
In LVH associated with AS, there is an increased production of collagen and a shift toward inhibition of collagen degradation.16–18 When compared with controls, myocardial biopsies of AS patients have higher expression of collagens and an up-regulation of tissue inhibitor of metalloproteinase-1 (TIMP1) and tissue inhibitor of metalloproteinase-2 (TIMP2) mRNA, favoring inhibition of collagen degradation, which significantly correlates with the degree of fibrosis.18 Experimental studies have described total regression of matrix metallopeptidase (MMP) and TIMP gene expression as well as an association between changes in LVMI and MMP/TIMP gene expression after corrective surgical therapy and LV hypertrophy regression.19 The renin-angiotensin-aldosterone system seems to be a key factor in this process. Mechanical stretch induces local production of angiotensin II, which in turn stimulates the release of multiple growth factors and cytokines from cardiac fibroblasts that act in an autocrine and paracrine fashion, affecting the progression of hypertrophy and remodeling.20–22
Noninvasive assessment of myocardial fibrosisThe noninvasive evaluation of the level of myocardial fibrosis has been object of several studies, using surrogate echocardiographic parameters or CMR imaging.
Cardiac magnet resonance imaging is currently the gold standard method for measuring LV wall thickness, mass, volume and EF. This technique shows structural changes in the myocardium, including replacement fibrosis with LGE and expansion of extracellular volume using T1 mapping.23 LGE is usually associated with more advanced forms of myocardial disease, worse systolic function and higher LV end-diastolic volumes,24,25 and, therefore, worse prognosis.5 A recent meta-analysis of 2032 patients found that LGE has a substantial value in all-cause mortality and cardiovascular mortality prediction.26 However, LGE has the limitation of only identifying regional differences in replacement myocardial fibrosis, and can miss diffuse interstitial fibrosis, which is an earlier event in disease progression. Nevertheless, T1 mapping, by measuring the expansion of the extracellular volume, reflects in part alterations in diffuse myocardial fibrosis, which is a reversible early form of fibrosis.13 Chin et al. showed, in a large prospective cohort, that the use of T1 mapping in combination with LGE leads to the categorization of patients in groups with different prognostic values.27 However, further studies are needed to demonstrate whether this technique can assess the transition from hypertrophy to HF in AS.
In severe AS, the degree of myocardial fibrosis is crucial in the transition from compensated hypertrophy to HF.4 As fibrosis worsens, LV filling pressures increase and, later on, EF decreases. However, EF is reduced only in end-stage disease since it is related to global radial function,28 and fibrotic changes in AS hypertrophic hearts are initially subendocardial and affect basal segments (where regional wall stress is highest). This will principally have an impact on longitudinal function, which is not well represented by EF.11 Mitral ring displacement and strain imaging seem to have overcome EF limitations in evaluation of early changes in systolic function. In a recent study, in patients with symptomatic AS and severe fibrosis, radial function was relatively preserved, while mitral ring displacement, a surrogate of overall longitudinal function of the septum, was reduced and predicted functional improvement.11 Furthermore, using strain imaging, evidence of subclinical systolic LV dysfunction with decreased LV strain and strain-rate can be seen in patients with severe AS and preserved LVEF.29,30
In our study, we used peak systolic annular velocity (Sm) to assess longitudinal function and found that patients with Sm<4.9 cm/s had worse survival and survival free of death or non-fatal cardiovascular hospitalization. Our data support the prognostic importance of assessing early parameters of systolic function in AS.
LimitationsThis was a single center study and the small size of our cohort may have precluded the identification of other predictors of clinical events. Moreover, the limited number of events in our study can result in an overfitted multivariate model. These limitations have been described by other groups with published results on this topic and who have included similar sample sizes. Obtaining biologic samples for histological analysis and conducting prospective studies with long-term follow-up can be challenging and only overcome by multicentric collaboration.
ConclusionsWe have confirmed the prognostic relevance of myocardial fibrosis in severe AS and extended this evidence to low risk patients with a less severe form of myocardial remodeling. Fibrosis is an ominous sign in AS in the continuous of myocardial structural changes and should be actively sought out for risk stratification, not only in asymptomatic patients with preserved EF, but also in symptomatic patients undergoing AVR. After AVR, the use of additional medical therapy modulating the renin-angiotensin system in patients with non-invasive evidence of fibrosis should be tested in large-scale randomized trials.
Funding sourcesThis work was supported by the Portuguese Foundation for Science and Technology (POCI/SAU-PIC/IC/82943/2007).
Conflicts of interestThe authors have no conflicts of interest to declare.