Patients with hypertrophic obstructive cardiomyopathy (HOCM) that remain symptomatic despite optimized medical therapy often undergo alcohol septal ablation (ASA). One of the most frequent complications is complete heart block (CHB), requiring a permanent pacemaker (PPM) in variable rates of up to 20% of patients. The long-term impact of PPM implantation in these patients remains unclear. This study aimed to evaluate the long-term clinical outcomes in patients who implant PPM after ASA.
MethodsPatients who underwent ASA at a tertiary center were consecutively and prospectively enrolled. Patients with previous PPM or implantable cardio-defibrillator were excluded from this analysis. Patients with and without PPM implantation after ASA were compared based on their baseline characteristics, procedure data and three-year primary endpoint of composite of all-cause mortality and hospitalization and secondary endpoint of composite of all-cause mortality and cardiac cause hospitalization.
ResultsBetween 2009 and 2019, 109 patients underwent ASA, 97 of whom were included in this analysis (68% female, mean age 65.2 years old). 16 patients (16.5%) required PPM implantation for CHB. In these patients, no vascular access, pacemaker pocket or pulmonary parenchyma complications were noted. The baseline characteristics of comorbidities, symptoms, echocardiographic and electrocardiographic findings were identical in the two groups, with higher mean age (70.6±10.0 years vs. 64.1±11.9 years) and lower beta-blocker therapy rate (56% vs. 84%) in the PPM group. Procedure-related data showed higher creatine kinase (CK) peaks in the PPM group (1692 U/L vs. 1243 U/L), with no significant difference in the alcohol dose. At three years after ASA procedure, there were no differences in the primary and secondary endpoints between the two groups.
ConclusionsPermanent pacemaker after ASA induced CHB do not affect long term prognosis in hypertrophic obstructive cardiomyopathy patients.
Doentes com miocardiopatia hipertrófica obstrutiva (MCHO) sintomáticos apesar de terapêutica médica otimizada são frequentemente submetidos a ablação septal alcoólica (ASA). O bloqueio atrioventricular (BAV) completo é uma complicação frequente, requerendo implantação de pacemaker definitivo em taxas variáveis que podem ir até 20% dos doentes. O impacto a longo prazo da implantação de pacemaker nestes doentes permanente mal-esclarecido. Esta análise pretende avaliar os outcomes clínicos a longo prazo nestes doentes.
MétodosDoentes consecutivos submetidos a ASA num centro terciário foram incluídos e seguidos prospetivamente. Pacientes com pacemaker ou cardiodesfibrilhador prévio foram excluídos desta análise. Os doentes com e sem implantação de pacemaker após a ASA foram caracterizados e comparados relativamente aos endpoints primário (composto de mortalidade e hospitalização por todas as causas) e secundário (composto de mortalidade por todas as causas e hospitalização de causa cardíaca) a três anos.
ResultadosEntre 2009 e 2019, 109 pacientes foram submetidos a ASA e 97 foram incluídos nesta análise (68% sexo feminino, idade media 65,2 anos). Implantaram pacemaker 16 doentes (16,5%) após a ASA por BAV completo. Não se registaram complicações vasculares, da loca ou parenquimatosas pulmonares. As características basais foram semelhantes entre os grupos, com maior idade média (70,6±10,0 anos versus 64,1±11,9 anos) e menor taxa de terapêutica beta-bloqueante (56% versus 84%) no grupo de pacemaker. Dados do periprocedimento revelaram maior pico de creatina cinase (CK) no grupo de pacemaker, sem diferença na dose de álcool usado. Aos três anos, os endpoints primário e secundário não mostraram diferenças entre os dois grupos.
ConclusãoA implantação de pacemaker definitivo por BAV completo induzido por ablação septal alcoólica não afeta o prognóstico de pacientes com MCHO.
Alcoholic septal ablation (ASA) is an alternative to surgical septal reduction therapy for symptomatic hypertrophic obstructive cardiomyopathy (HOCM) patients, despite medical therapy. Overall clinical response is favorable.1–3 One of the most frequent complications is complete heart block (CHB) which occurs typically within the first 72 hours post-procedure.4,5 Incidence varies from 14 to 38%.6–8 Pre-existing bundle branch block, first degree AV block, being female, increased age, volume of ethanol injection and injection of two or more septal perforators are reported contributing factors.7 In some patients, AV conduction is transitory and compromised due to peri-myocardial infarction inflammatory response and others suffer permanent conduction tissue lesions provoked directly by the alcohol. Nowadays, watchful waiting and clinical scores enable the patients to be selected properly for PPM, although there is a chance of AV conduction recovery afterwards. PPM implantation occurs in up to 20% of the patients. Patients requiring PPM implantation after ASA have similar New York Heart Association (NYHA) functional class improvement, septal thickness reduction and left ventricle outflow tract (LVOT) reduction when compared to patients who do not require PPM placement.4 In the Euro-ASA registry, a multinational ASA study including 1275 patients, survival rates at one, five and 10 years after ASA were 98%, 89% and 77%, respectively.8 In that analysis, the only established independent predictors of all-cause mortality were age at ASA and pre-procedural septum thickness and NYHA class.9 The long-term impact of PPM implantation in these patients remains unclear.
ObjectivesThis study aimed to evaluate long-term pacemaker dependency in patients with PPM after ASA and to assess the long-term impact of PPM.
MethodsPatient selectionRetrospective analysis was performed of consecutive HOCM patients who underwent ASA at a single tertiary center. The indications for ASA in these patients were dyspnea NYHA class III or IV, CCS class II or III angina or exertional syncope in association with significant dynamic LVOT obstruction (defined as >50 mmHg at rest and/or >70 mmHg with provocation). Patients with previous permanent pacemaker or implantable cardio-defibrillator were excluded from this analysis.
Procedure protocolThe procedure was performed as described previously by Fiarresga et al.10 1.7–3 cm3 of 98% dehydrated ethanol was used for ASA. All patients were admitted to the cardiac intensive care unit (ICU) with a temporary pacemaker device placed during the procedure as a precaution for the development of a high-grade atrioventricular (AV) block, usually during 24–48 hours. A risk score for AV block was used to predict pacemaker dependency and select the patients for prolonged monitoring and/or PPM implantation, as described by Faber et al.11 All patients without previous PPM had continuous telemetry monitoring and daily 12-lead electrocardiogram until discharge. The risk score was applied at 24 h after ASA in all patients who did not develop persistent complete AV block. This score stratifies patients into three categories of pacemaker dependency risk (low, intermediate, and high risks). Patients with a low-risk score at 24 h after ASA were transferred to a cardiology ward under continuous telemetry monitoring; intermediate risk patients underwent prolonged monitoring in the ICU for up to 4–5 days plus corticoid therapy (prednisolone 1 mg/kg/day for four days) to assess the reversibility of AV conduction disturbances. In high-risk patients, the PPM indication was established on the second day after ASA. All the patients underwent a transthoracic echocardiogram before discharge and were followed up periodically. Patients who received a PPM had device analysis at one month after implantation and then annually thereafter.
Follow-up and outcomesThe included patients were divided in two groups: PPM placement after ASA and those who do not undergo the procedure) and were followed for a minimum period of three years. The baseline characteristics, procedure data and outcomes were noted and compared. The long-term pacing rates in the PPM group and the PPM implantation rate in the no-PPM group during follow-up were also evaluated. The primary endpoint was composite all-cause mortality and all-cause hospitalization, and the secondary endpoint was composite all-cause mortality and hospitalization due to a cardiac cause.
Statistical analysisContinuous variables are presented as mean value and standard deviation and categorical variables in absolute and relative values. The comparative analysis was performed using the Student's T test and chi-square test for the categorical variables. The results were presented as odds ratio (OR), 95% confidence interval (CI) and p-value and endpoints were also displayed in Kaplan-Meier curves. Statistical analysis was performed using SPSS version 25.0.
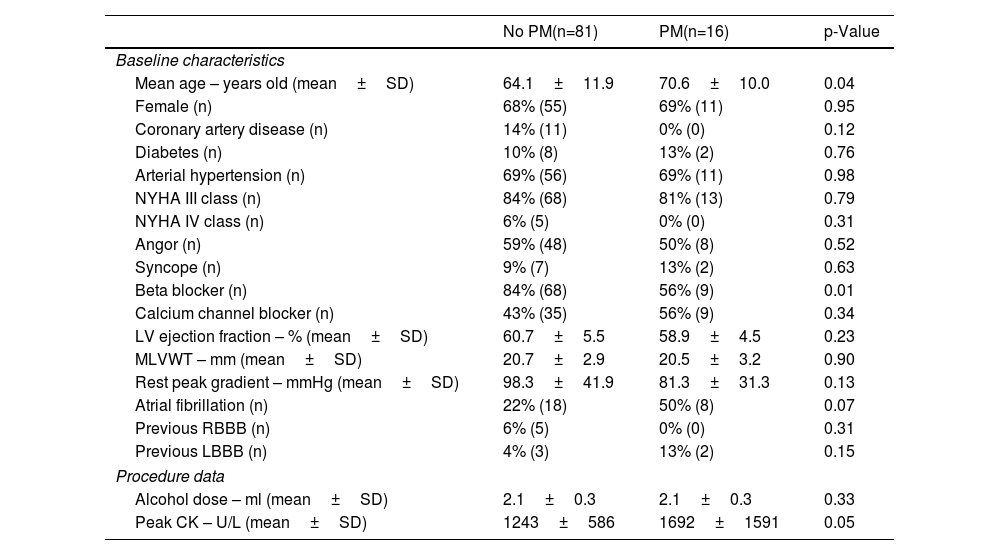
ResultsPopulation baseline characteristicsBetween 2009 and 2020, 109 consecutive patients underwent ASA. 97 patients were included in this analysis (68% female, mean age 65.2 years old). 16 patients (16.5%) required PPM implantation for CHB. In these patients, no vascular access, pacemaker pocket or pulmonary parenchyma complications were noted. The baseline characteristics of comorbidities, symptoms, echocardiographic and electrocardiographic findings (Table 1) were identical in the two groups. There were statistically significant differences in the mean age (70.6 years old in the PPM group vs. 64.1 years old in the non-PPM group) and in the beta-blocker therapy rates prior to the intervention (56% in the PPM group vs. 84%). Procedure-related data showed higher creatine kinase (CK) peaks in the PPM group (1692 U/L vs. 1243 U/L, p=0.05), with no significant differences in the alcohol dose (2.1 ml in both groups, p=0.33).
Baseline characteristics and procedure data.
| No PM(n=81) | PM(n=16) | p-Value | |
|---|---|---|---|
| Baseline characteristics | |||
| Mean age – years old (mean±SD) | 64.1±11.9 | 70.6±10.0 | 0.04 |
| Female (n) | 68% (55) | 69% (11) | 0.95 |
| Coronary artery disease (n) | 14% (11) | 0% (0) | 0.12 |
| Diabetes (n) | 10% (8) | 13% (2) | 0.76 |
| Arterial hypertension (n) | 69% (56) | 69% (11) | 0.98 |
| NYHA III class (n) | 84% (68) | 81% (13) | 0.79 |
| NYHA IV class (n) | 6% (5) | 0% (0) | 0.31 |
| Angor (n) | 59% (48) | 50% (8) | 0.52 |
| Syncope (n) | 9% (7) | 13% (2) | 0.63 |
| Beta blocker (n) | 84% (68) | 56% (9) | 0.01 |
| Calcium channel blocker (n) | 43% (35) | 56% (9) | 0.34 |
| LV ejection fraction – % (mean±SD) | 60.7±5.5 | 58.9±4.5 | 0.23 |
| MLVWT – mm (mean±SD) | 20.7±2.9 | 20.5±3.2 | 0.90 |
| Rest peak gradient – mmHg (mean±SD) | 98.3±41.9 | 81.3±31.3 | 0.13 |
| Atrial fibrillation (n) | 22% (18) | 50% (8) | 0.07 |
| Previous RBBB (n) | 6% (5) | 0% (0) | 0.31 |
| Previous LBBB (n) | 4% (3) | 13% (2) | 0.15 |
| Procedure data | |||
| Alcohol dose – ml (mean±SD) | 2.1±0.3 | 2.1±0.3 | 0.33 |
| Peak CK – U/L (mean±SD) | 1243±586 | 1692±1591 | 0.05 |
CK: creatine kinase; IVS: interventricular septum; LBBB: left bundle branch block; LV: left ventricle; NYHA: New York Heart Association; PM: pacemaker; RBBB: right bundle branch block.
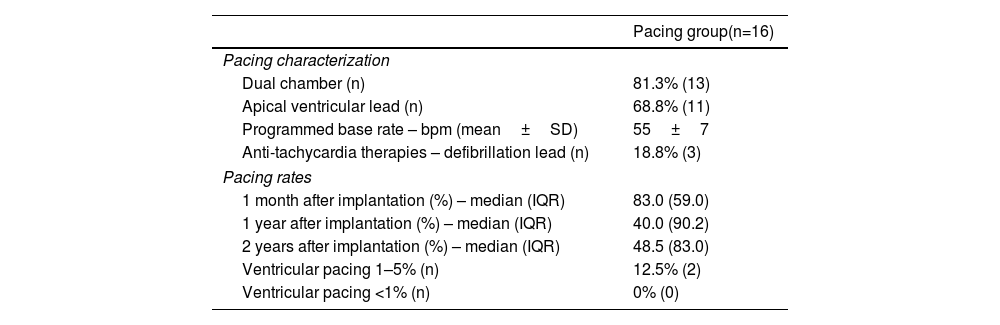
In all patients, implantation of a device during hospitalization after ASA was due to AV conduction disturbances. However, in three out of 16 patients (19%) a defibrillation lead was placed in the right ventricle (RV) to perform anti-tachycardia therapies as primary prevention. 13 patients (81%) implanted a dual chamber pacemaker. In the remaining three, a single lead was placed due to permanent atrial fibrillation. Regarding the site of RV pacing, the lead was placed in an apical position in 11 patients (69%) and septal in five patients (31%). The mean programmed base rates was 55±7 bpm. Minimal pacing algorithms were used in all patients with a dual chamber pacemaker to reduce RV pacing in patients with intermittent AV conduction disturbances and enable longer intrinsic AV delay in patients with first degree AV block.
In the PPM group, the median (inter-quartile range) pacing rates (Table 2) at one month, one year and two years were 89.0 (59.0), 40.0 (90.2) and 48.5 (83.0), respectively. Two patients (12.5%) had 1–5% pacing and none had pacing <1% at two years.
Implanted devices characterization, programming, and long-term pacing rates.
| Pacing group(n=16) | |
|---|---|
| Pacing characterization | |
| Dual chamber (n) | 81.3% (13) |
| Apical ventricular lead (n) | 68.8% (11) |
| Programmed base rate – bpm (mean±SD) | 55±7 |
| Anti-tachycardia therapies – defibrillation lead (n) | 18.8% (3) |
| Pacing rates | |
| 1 month after implantation (%) – median (IQR) | 83.0 (59.0) |
| 1 year after implantation (%) – median (IQR) | 40.0 (90.2) |
| 2 years after implantation (%) – median (IQR) | 48.5 (83.0) |
| Ventricular pacing 1–5% (n) | 12.5% (2) |
| Ventricular pacing <1% (n) | 0% (0) |
IQR: interquartile range.
In the group without PPM, five patients (6.2%) required PPM implantation at a later date, during the follow-up (six months, one year, one year, two years and three years after ASA), all of them for AV conduction disturbances. No other patients received an ICD during the follow-up period under analysis.
OutcomesIn the 16 patients who implanted PPM after ASA during hospitalization, no vascular, pocket-related or pulmonary complications were recorded. During the three-year follow-up, there were no recorded pocket-related, device or lead complications.
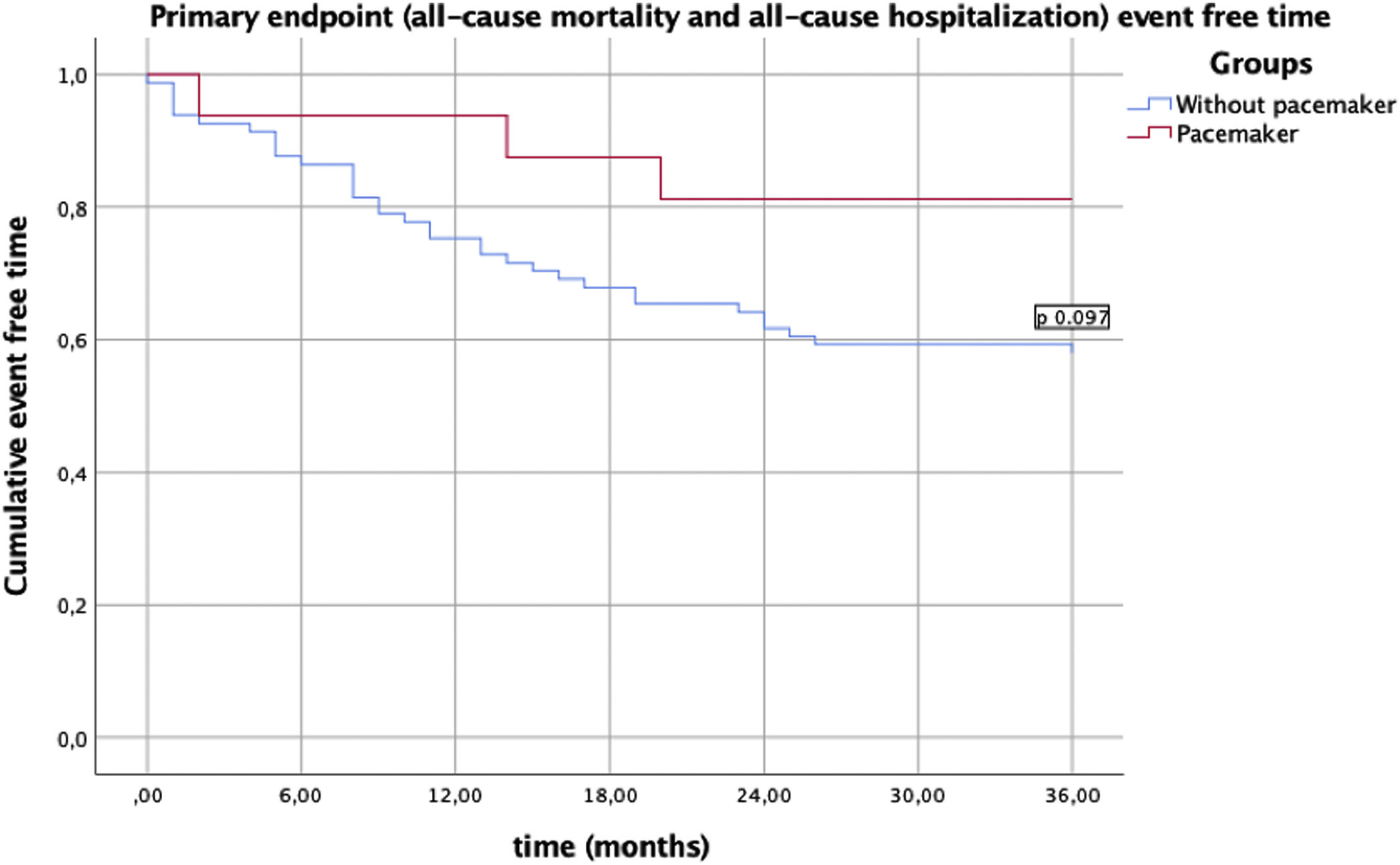
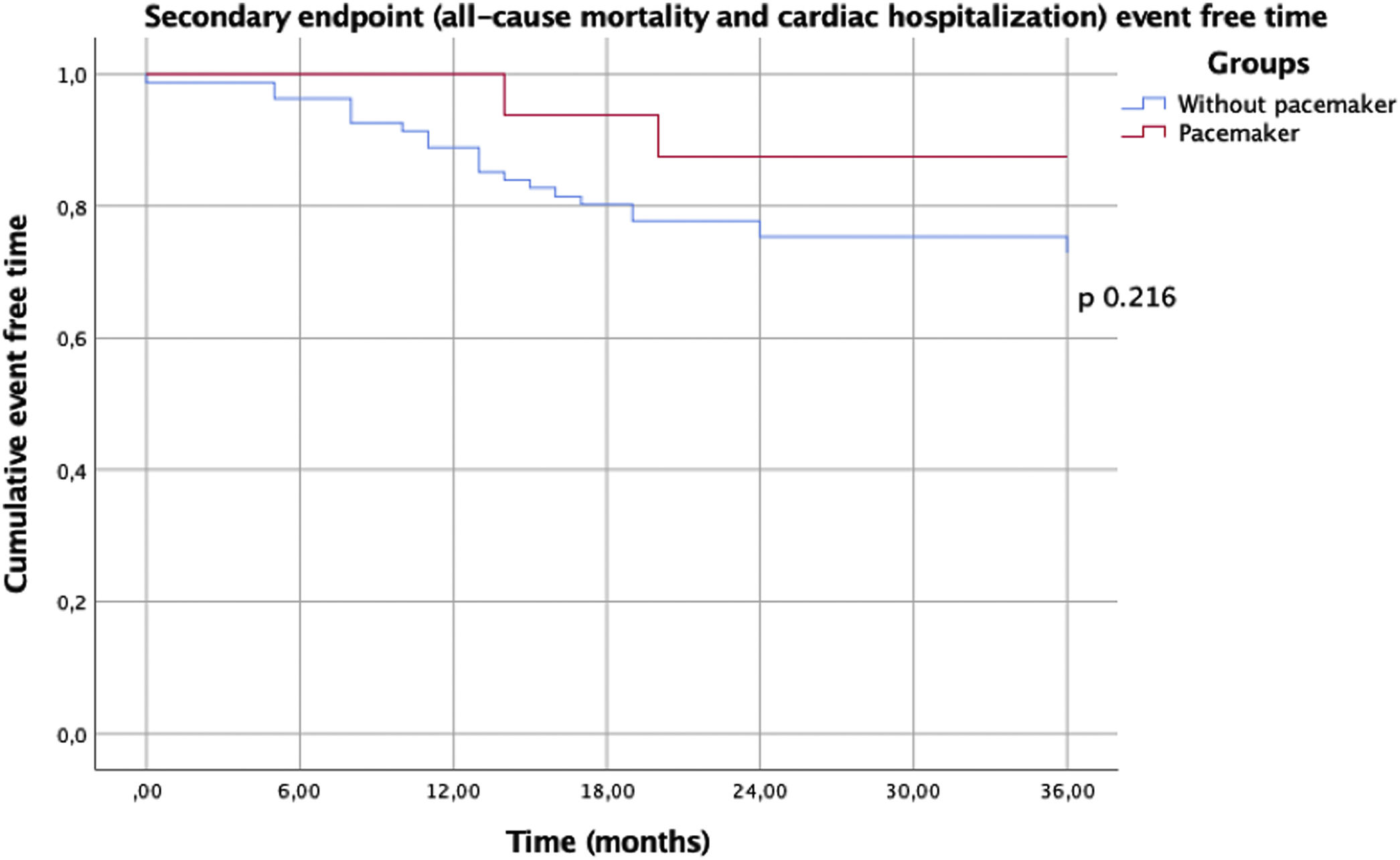
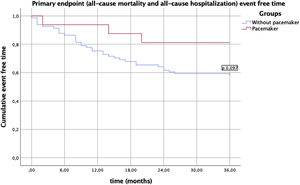
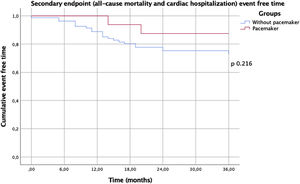
In this analysis, no in-hospital deaths occurred during hospitalization for the procedure. There were no statistically significant differences in the two groups regarding one year mortality or re-hospitalization (Table 3). There was no difference in the primary endpoint of composite all-cause mortality and all-cause hospitalization (Figure 1) between the two groups, showing, however, a trend toward a lower event rate in the PM group (p=0.09). This difference appears to be attributable to a lower rate of all-cause re-hospitalization (19% vs. 43%, p=0.07). There was no difference in the secondary endpoint of composite all-cause mortality and hospitalization due to a cardiac cause (Figure 2) between the two groups (p=0.22).
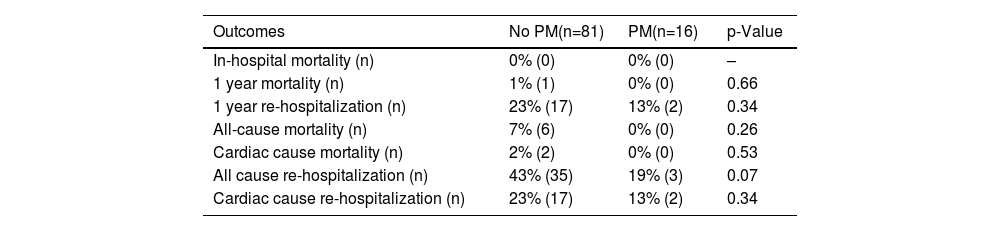
Outcomes comparison between the two groups, including separate component endpoints.
| Outcomes | No PM(n=81) | PM(n=16) | p-Value |
|---|---|---|---|
| In-hospital mortality (n) | 0% (0) | 0% (0) | – |
| 1 year mortality (n) | 1% (1) | 0% (0) | 0.66 |
| 1 year re-hospitalization (n) | 23% (17) | 13% (2) | 0.34 |
| All-cause mortality (n) | 7% (6) | 0% (0) | 0.26 |
| Cardiac cause mortality (n) | 2% (2) | 0% (0) | 0.53 |
| All cause re-hospitalization (n) | 43% (35) | 19% (3) | 0.07 |
| Cardiac cause re-hospitalization (n) | 23% (17) | 13% (2) | 0.34 |
PM: pacemaker.
In this analysis, we describe a reasonable cohort of patients who underwent ASA in whom the impact of post-ASA pacemaker implantation was assessed. The registered pacemaker implantation rate of 16.5% is aligned with the rates described in literature and previous studies.5–7 The baseline characteristics were balanced in the two groups, with exception of a higher age in the PPM group, which may be explained by a lower threshold for device implantation in older patients. This can be attributed to a reduced need of future device re-interventions and associated complications, including pocket infection and hematoma and a lower beta-blocker therapy rate in the PPM group. We believe that this can be explained by the presence of previous conduction disease. In spite of the amount of alcohol injected and the number or injected septal perforators having been previously described as predictors of CHB after ASA,6 in our analysis there was no difference in the average alcohol volume between the two groups. However, a higher CK peak in the PPM group was noted, which could potentially indicate a larger extent of infarction area. No pacemaker-related complications were observed during the follow-up period. The registered pacing rates show that all devices were adequately implanted and that, in fact, most of the patients who do not resolve their CHB in the first days, remain dependent on pacing in long-term analysis. These data enhance the use of a proper AV block risk score, as it enables early transfer to a cardiology ward/discharge of low-risk patients and avoids unnecessary prolonged hospitalization. On the other hand, it enables the identification of patients at high risk for pacemaker dependency, which helps in the decision regarding early permanent pacemaker implantation after ASA.
Regarding the outcomes, although PPM implantation had not been described in previous studies as a predictor of poor outcomes in HOCM patients, the real impact of PPM implantation in HOCM patients in terms of clinical events has never been evaluated. Globally, chronic RV apical pacing has been associated with LV dysfunction (RV pacing-induced cardiomyopathy), atrial fibrillation, increased risk of heart failure.12–15 However, in HOCM patients, pacing has been proposed as a potential beneficial therapy for HOCM patients, as it induces asynchronous septal activation, delaying septal thickening, and reducing LV hypercontractility with potential benefits in LVOT obstruction.16 Small randomized trials17,18 and Cochrane analysis19 recognize the benefit of pacing in LVOT obstruction reduction, although with improvement in exercise capacity, morbidity, and mortality. Also, HOCM patients often present with supranormal LV systolic function. This analysis provides insights suggesting that the hospitalization rate and all-cause mortality is not different between patients who implant PPM after ASA and those who do not require PPM implantation, and that AV block after ASA does not translate into a worse prognosis. We believe the lack of deleterious effect of pacing in this cohort can then be explained by the particularity that HOCM patients usually have abnormal elevated LV systolic function and that this physiopathologic feature attenuates the deleterious effect of pacing. Furthermore, the analyzed period of follow-up could be short to assess the impact of pacing on major outcomes, such as cardiac hospitalization and mortality.
LimitationsThis study has some limitations such as the reduced sample size and a reduced event rate in the follow-up period, mainly regarding mortality, which does not allow a proper characterization of this component endpoint. Also, the follow-up period used does not encompass potential hospitalizations and complications in the PPM group associated with replacement of the device's generator.
ConclusionsThe PPM implantation rate after ASA and the follow-up pacing dependency in our cohort enhances the importance of proper patient selection for PM implantation after ASA and the benefit of using a dedicated AV block risk score in these patients. The long-term impact analysis suggests that the outcomes in patients who implant PPM after ASA are similar those who do not. Further studies and registries with larger samples and extended follow-ups will help to confirm these data.
Conflicts of interestThe authors have no conflicts of interest to declare.