Myocardial infarction with non-obstructive coronary arteries (MINOCA) is responsible for 10% of myocardial infarctions. Previously, patients were thought to have good prognosis, but evidence-based management and treatment strategies were scarce. Today, researchers and physicians recognize MINOCA as a condition with non-trivial mortality and morbidity. Therapeutic strategies are highly dependent on the underlying disease mechanism in each patient. However, to reach a diagnosis of MINOCA, a multimodal approach is required and, even with an optimal work-up, the cause remains unknown in 8–25% of patients. Research has been growing and position papers from the European Society of Cardiology (ESC) and the American Heart Association/American College of Cardiology have been published, and MINOCA has been included in the more recent ESC guidelines on myocardial infarction. Nonetheless, some clinicians still assume that the absence of coronary obstruction excludes the possibility of acute myocardial infarction. Therefore, in the present paper, we aim to compile and present the available data on the etiology, diagnosis, treatment, and prognosis of MINOCA.
O enfarte agudo do miocárdio sem obstrução das artérias coronárias (Minoca) é responsável por 10% de todos os enfartes agudos do miocárdio. Anteriormente, pensava-se que os pacientes teriam bom prognóstico e a orientação do doente e estratégias de tratamento baseadas em evidência eram escassas. Atualmente, investigadores e clínicos reconhecem que a mortalidade e morbilidade desta condição não é insignificante. As estratégias terapêuticas são altamente dependentes do mecanismo subjacente da doença em cada doente. Contudo, para fazer um diagnóstico de Minoca, uma abordagem multimodal é necessária e, apesar de um estudo diagnóstico aprofundado, a causa permanece desconhecida em 8-25% dos pacientes. Recentemente, a investigação nesta área tem crescido e foram publicados artigos de consenso por parte da European Society of Cardiology (ESC) e da American Heart Association (AHA)/American College of Cardiology (ACC) e o Minoca foi integrado nas recomendações mais recentes sobre enfarte agudo do miocárdio da ESC. Não obstante, alguns clínicos ainda assumem que a ausência de obstrução coronária exclui a possibilidade de enfarte agudo do miocárdio. Portanto, esta revisão tem como objetivo compilar e apresentar a informação disponível sobre etiologia, diagnóstico, tratamento e prognóstico de Minoca.
Myocardial infarction (MI) in patients without significant coronary artery disease (CAD) has been reported since 1939, when Harry Gross and William Sternberg described its occurrence as “not rare”.1 Analysis of the CRUSADE initiative, a nationwide registry in the USA for non-ST-elevation acute coronary syndromes published in 2015,2 showed that 10% of these patients had non-obstructive CAD.3 More recent studies confirm this finding, showing a prevalence of 5–15%,4–12 although the percentage is dependent on the proportion of MI patients who undergo coronary angiography and on the cardiac troponin assays used.9
Initially, MI with normal coronary arteries (MINCA) was the term used to describe patients with MI with unobstructed coronary arteries, but this was later replaced by MI with non-obstructive coronary arteries (MINOCA), so that MINOCA includes both MINCA patients and those with evidence of low or moderate levels of atherosclerosis.12 It is thought that half of MINOCA patients have completely angiographically normal coronary arteries, while the remainder show some degree of irregularities.4 MINOCA is a heterogeneous entity8,12–14 with a broad range of underlying pathophysiological mechanisms and disease severity. Although it was initially an umbrella term, including patients with acute coronary syndrome and unobstructed coronary arteries, acute myocarditis or Takotsubo syndrome (TTS), since the publication of the 2020 European Society of Cardiology (ESC) guidelines on non-ST-elevation MI (NSTEMI)15 the term MINOCA should be reserved for patients presenting with MI with unobstructed coronary arteries only.
MINOCA may present with or without ST-segment elevation,4,12 with about 66% of cases presenting as NSTEMI,4 which makes ST-segment elevation MI (STEMI) less frequent in this population than in MI due to coronary artery disease (MI-CAD). Troponin is less elevated in MINOCA patients than in obstructive MI12,16 and MINOCA shows a circadian and circaseptan rhythm, with increased risk in the early morning and on Mondays.17
It is important to recognize that this entity, as well as being frequent, although previously assumed to be benign,18–21 is in fact associated with significant mortality and morbidity.10,13,20,22 All-cause 30-day mortality is 1–3%4,13 and all-cause mortality at one-year follow-up ranges between 3% and 6%.4,10,13,23
Although the literature on this subject is growing rapidly, some clinicians still believe that the absence of obstructive CAD excludes MI,12 posing “a diagnostic and therapeutic dilemma”,24 and consequently many MINOCA patients are discharged without an appropriate therapeutic strategy, which should be targeted according to the underlying cause.15
Younger age and over-representation of females, compared to MI-CAD patients, are consistent findings in MINOCA groups.12,15,22,25,26 Data show that women have 4.8 times higher odds of presenting with MINOCA than men, and non-white patients have 1.5 higher odds of presenting with MINOCA than white patients.22 A lower prevalence of dyslipidemia in MINOCA compared to MI-CAD patients also seems to be a consistent finding,4,12,25 and although not consistently reported in all studies in this review, large studies and guidelines report fewer traditional risk factors like smoking and diabetes in MINOCA than in CAD patients.15,22,27
Since this is an expanding subject, this paper aims to critically review up-to-date scientific papers and guidelines on the management and treatment of these patients and to highlight areas of conflicting evidence.
MethodsArticles included were identified in PubMed by searching for the term “MINOCA” as of August 7, 2021, and 280 articles were found. After abstract screening, 161 were deemed possibly relevant, of which 71 were selected. When considered relevant to the discussion, additional research on specific areas was performed and the references added. Only articles in English were included.
EtiologyThe definition of MINOCA as an entity is based on the presumption that unobstructed coronary arteries can be found at angiography. This does not exclude an atherothrombotic etiology, as thrombosis is a dynamic phenomenon and atherosclerotic plaque can be non-obstructive, ruptured, or eroded.28,29
Plaque disruption is the mechanism behind non-obstructive atherosclerotic MI. Plaque burden in MINOCA patients is similar to that in healthy control subjects.30 Plaque disruption can occur in the form of plaque rupture, plaque erosion or calcific nodules, and can be found in more than a third of MINOCA patients.12,30 Plaque disruption can trigger thrombus formation with distal embolization and MI, superimposed vasospasm, and even transient complete thrombosis with spontaneous thrombolysis, or a combination of all these processes.12,30,31 The distinction between plaque rupture and plaque erosion is better made by intracoronary imaging, as computed tomography (CT) angiography can only suggest its presence.12 Calcified nodules are the least common cause of plaque disruption, and are typically more common in older patients.12
There are various non-atherosclerotic causes for MINOCA. Epicardial vasospasm can occur in response to exogenous substances or spontaneously,12,30 and inducible vasospasm can be found in 28% of patients.4 In a study including only MINOCA patients with suspected coronary vasomotor abnormalities, 46% were confirmed to have vasospasm abnormalities, with 65% of these having epicardial vasospasm and 35% microvascular vasospasm.32 In the MINOCA population, this inducible vasospasm is persistent over time, unlike in MI-CAD patients,4 possibly reflecting an underlying vasospastic predisposition.
The microcirculation accounts for roughly 70% of coronary resistance, yet it is not easily evident through angiography.12 It can therefore be hypothesized that when no epicardial coronary is obstructed, there is a chance that one or more small-diameter obstructed vessels may be the cause of infarction.
Coronary microvascular dysfunction (CMD) can contribute to MINOCA and can be classified as endothelium-dependent or endothelium-independent.12 CMD can cause ischemia or can result from myocardial injury.12 It is more common in women (especially postmenopausal23) and patients with cardiovascular risk factors such as diabetes, hypertension, smoking, dyslipidemia and older age, and can be detected in up to 50% of patients with chest discomfort and non-obstructive CAD.12,23
Coronary thrombosis/embolism can explain a MINOCA presentation if it occurs in the microvasculature or if there is partial or total lysis of epicardial coronary thrombus or emboli.12,30,33 These thrombotic events can occur in the presence or absence of a hypercoagulable state, such as inherited or acquired thrombophilia disorders, or conditions like atrial fibrillation and valvular heart disease.12,23,30 In consequence, it is difficult to identify the percentage of MINOCA patients with coronary thrombosis/embolism, although most authors think its prevalence is low.30
Spontaneous coronary artery dissection (SCAD) is usually an uncommon cause of MINOCA, however, it is particularly important in certain groups such as women aged under 50 years with MI,12,23 fibromuscular dysplasia, pregnancy and the peripartum period, in the absence of typical cardiovascular risk factors.12,23 Most patients with SCAD present some degree of blood flow obstruction, but in some cases, arteries can appear normal or near normal at angiography, therefore, with wider adoption of intracoronary imaging, it is thought that SCAD will become increasingly recognized. The estimated prevalence of SCAD in patients with acute coronary syndromes ranges between 2% and 4%, although in young female patients it can account for 35% of all acute coronary syndromes.12,23 The prevalence of SCAD in MINOCA patients is still unknown.12 Emotional stress, among other factors, can provoke an acute SCAD event,23 and one study found that 59% of patients recalled physical and/or emotional distress leading to admission for MINOCA.25 Another study34 supports this finding, with 71% of MINOCA patients acknowledging emotional stress versus 32% in the MI-CAD group, and this is particularly relevant since this study excluded TTS patients from the MINOCA group. There are also case reports of emotional distress leading directly to MINOCA, although no SCAD was found; these cases are hypothesized to have occurred in the setting of intense catecholamine release possibly triggering coronary vasospasm,35 even though coronary thrombosis/embolism cannot be ruled out or confirmed. Alternatively, emotional stress could promote cardiac instability and increase the risk of arrhythmias and cardiac arrest. Additionally, stress as a trigger for MINOCA is supported by the finding of circadian and circaseptan patterns as mentioned above.35
Finally, there are other mechanisms that can lead to MINOCA, including other forms of type 2 MI, such as brady- or tachyarrhythmias, anemia and hypotension.12,30 Identifying these as a cause of MI may be difficult. Therefore, diagnosis is made when a plausible cause exists and in the absence of clinical, angiographic or invasive imaging findings supporting a different diagnosis.12
DiagnosisDiagnosing MINOCA can be a challenge and even with optimal work-up the underlying cause remains unknown in up to 25% of patients.15,30,36 Clinical history, electrocardiogram (ECG), echocardiography, cardiac biomarkers, and coronary angiography with left ventricular (LV) angiography are the first-level assessment methods.23,28 Diagnostic criteria for MINOCA were proposed in a 2016 position statement from the ESC,30 based on the Third Universal Definition of Myocardial Infarction. In their definition, patients with myocarditis, TTS and other non-ischemic conditions were to be diagnosed as having MINOCA. However, an American Heart Association (AHA) position paper from 201912 updated the MINOCA diagnostic criteria according to the updated Fourth Universal Definition of Myocardial Infarction,37 which now excludes myocarditis, TTS, cardiomyopathies and other entities from the final diagnosis of MINOCA.12,15,37
Recommendations for the use of diagnostic tools in patients with MI have changed over time. The 2017 ESC STEMI guidelines28 only briefly addressed the diagnostic algorithm for MINOCA, suggesting that further invasive or non-invasive diagnostic methods could be necessary, with insufficient data to give a class of recommendation. However, in the 2020 ESC NSTEMI guidelines15 and later in the 2021 American College of Cardiology (ACC)/AHA chest pain guidelines,38 cardiac magnetic resonance (CMR) received a class Ib indication to further investigate unclear causes of ACS with non-obstructive coronary arteries.
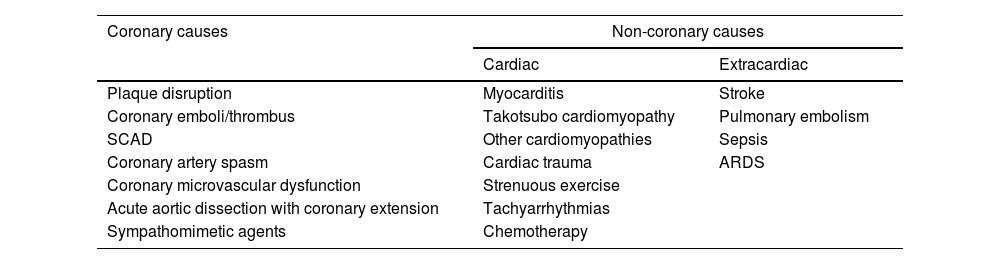
In addition to coronary causes, there are other clinical entities that can present with acutely elevated troponins30,39 (Table 1). Interpretation of cardiac biomarkers must therefore consider the clinical context, since release of cardiac troponin reflects myocardial injury and necrosis and is not a pathognomonic finding for MI.30 It is also important to emphasize that MINOCA patients can present with ST-segment elevation (17–33%) or ST-segment depression, as well as no ST-segment changes.40 Moreover, in these patients only slight ST-segment abnormalities are often detected.16
Potential causes for acutely elevated troponin levels.
| Coronary causes | Non-coronary causes | |
|---|---|---|
| Cardiac | Extracardiac | |
| Plaque disruption | Myocarditis | Stroke |
| Coronary emboli/thrombus | Takotsubo cardiomyopathy | Pulmonary embolism |
| SCAD | Other cardiomyopathies | Sepsis |
| Coronary artery spasm | Cardiac trauma | ARDS |
| Coronary microvascular dysfunction | Strenuous exercise | |
| Acute aortic dissection with coronary extension | Tachyarrhythmias | |
| Sympathomimetic agents | Chemotherapy | |
ARDS: acute respiratory distress syndrome; SCAD: spontaneous coronary artery dissection.
The 2020 ESC NSTEMI guidelines15 suggest that, after an initial angiogram showing no obstructive disease and abnormal troponin results in a patient with ischemic signs and symptoms, LV function should be promptly assessed, through LV angiography, depending on renal function, or echocardiography.
Echocardiography is useful to assess wall motion abnormalities, which could be indicative of a specific coronary artery territory or suggestive of TTS,15,24 and can provide additional diagnoses. Transesophageal echocardiography may be also used when thromboembolism is suspected (due to intracardiac clots, shunts or valvular vegetations).41
CMR is useful as it helps in the differential diagnosis of TTS and myocarditis, or by confirming MI non-invasively.15,42 A third of patients identified as having MINOCA within the older MINOCA criteria have myocarditis, a condition well diagnosed by CMR.4,15
CMR can identify the underlying cause of MINOCA in up to 87% of patients,15 especially when it is performed early, at patient admission.28,43,44 A study including 204 MINOCA patients showed that the diagnostic value and clinical impact of CMR were highest when performed within two weeks of presentation, with a higher diagnostic yield (84% vs. 57%, p<0.0001), demonstrating the importance of imaging myocardial damage before healing occurs (particularly relevant for the diagnosis of potentially reversible conditions like myocarditis and TTS).44 Furthermore, CMR had a significant clinical impact in 66% of patients, with a new diagnosis being made in 54% (23% of patients were reclassified as MINOCA) and changes in management (including alterations in length of stay, medical therapy and invasive procedures) in 41%.44 The diagnostic yield of early CMR (within two weeks) is further increased when combined with cardiac biomarkers (peak troponin T>211 ng/l), leading to higher diagnostic pick-up rates (up to 94%).45
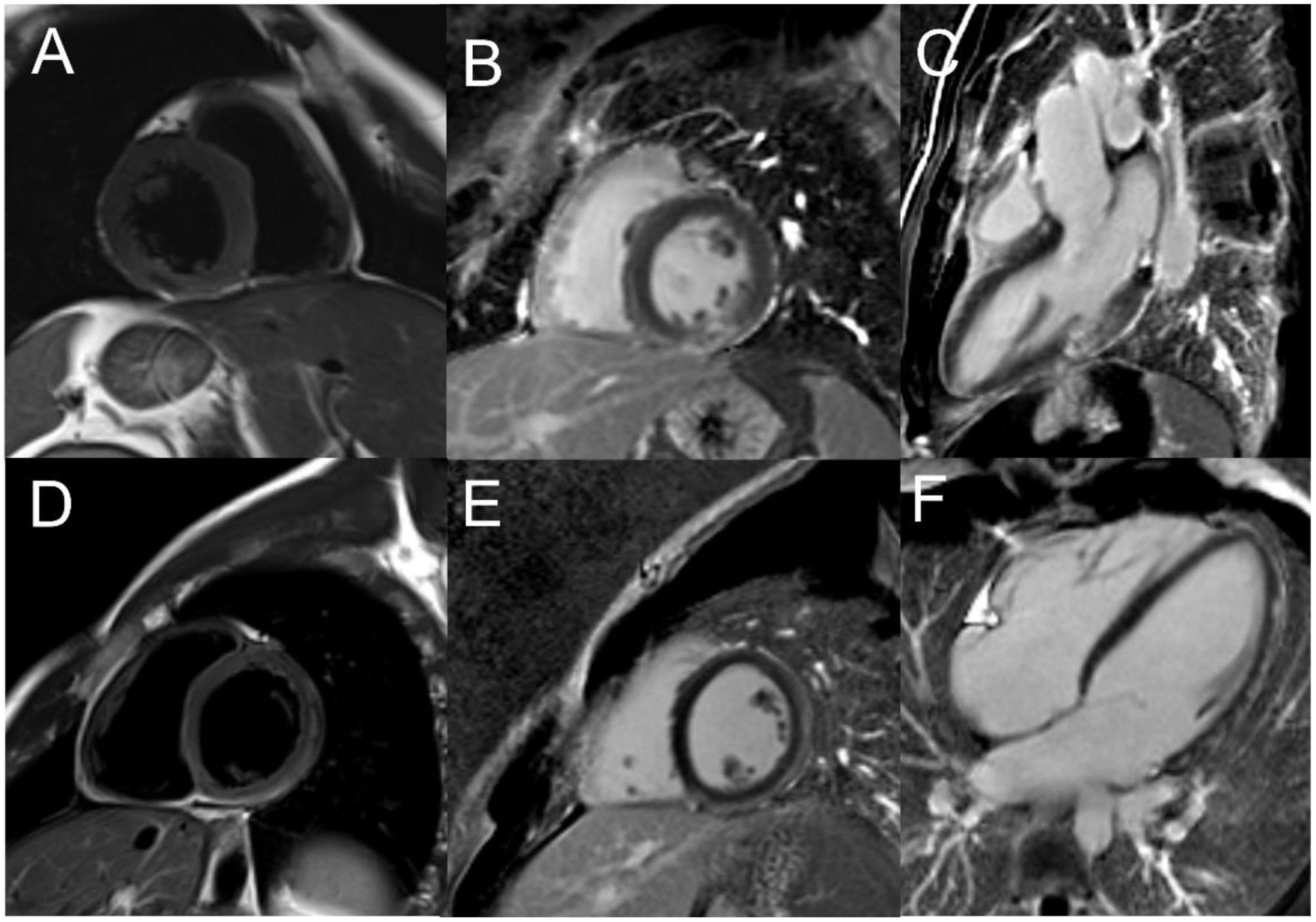
Late gadolinium enhancement (LGE) enables quantification of myocardial damage, detecting as little as 1 g of infarcted myocardium.30 LGE in the subendocardium indicates an ischemic injury (although not identifying the mechanism itself), while a subepicardial location is suggestive of cardiomyopathy or myocarditis,15,30 and the absence of significant LGE with edema and associated specific wall motion abnormalities is indicative of TTS15 (Figure 1). However, absence of myocardial necrosis on CMR does not exclude a MINOCA diagnosis, as this has been reported in patients with MINOCA.12
Role of cardiac magnetic resonance in the diagnostic approach of patients with myocardial infarction with non-obstructive coronary arteries (MINOCA). (A–C) Cardiac magnetic resonance (CMR) imaging of a 38-year-old male patient admitted with MINOCA. Cine imaging showed akinesia of the basal segment of the inferior wall. T2 (A) and late gadolinium enhancement (LGE) (B and C) sequences revealed recent almost transmural focal mid-cavity inferior infarction. A patent foramen ovale (PFO) was found and an embolic infarction was assumed. PFO closure was subsequently performed; (D–F) CMR imaging of a 39-year-old male patient admitted with MINOCA. T2 sequences (D) showed myocardial edema of the inferior and lateral walls with corresponding subepicardial LGE (E and F) in the same segments, thus enabling a diagnosis of myocarditis.
Coronary CT angiography (CCTA) is helpful in the diagnosis of MINOCA, since it can suggest ischemia or infarction by demonstrating perfusion defects and reveal plaque events (ulceration or fissures) as well as plaque morphology and composition, and can detect SCAD (but with lower resolution than intracoronary imaging).41,46 CCTA can likewise be useful in the follow-up of SCAD patients, avoiding repeated invasive angiography and the at least theoretical risk of further dissection, as long as the dissection could be observed on initial imaging.41
The perivascular fat attenuation index (pFAI), a CCTA marker of inflammation, can also aid in the differential diagnosis of MINOCA, myocarditis and TTS. In a small study, MINOCA patients showed more pronounced coronary inflammation in the acute phase (within one week), but this tended to disappear rapidly, whereas in myocarditis and TTS the pFAI values remained altered for longer.47
Intracoronary imaging, in the form of intravascular ultrasound (IVUS) or optical coherence tomography (OCT), provides precise assessment of coronary anatomy and plaque characteristics, and is useful when thrombus, plaque rupture, plaque erosion or SCAD are suspected but not observed on angiography.12,15,30,31,48 Around one in three patients has plaque disruption on assessment, and this proportion may be even greater since OCT (a higher-resolution imaging method) is not always used, and IVUS cannot identify plaque erosion.12,36,49 OCT is also the preferred method when SCAD is suspected, due to its higher resolution, although it may be associated with contrast-induced hydraulic extension of the dissection.12 These techniques are particularly useful when combined with CMR, confirming the value of multimodality imaging. In the HARP study,50 OCT and CMR were used in a group of women to assess mechanisms of MINOCA. Using a comprehensive imaging protocol, the authors found that OCT was able to identify the culprit lesion in 46% of patients (most commonly plaque rupture, intraplaque cavity or layered plaque) and CMR revealed abnormalities with an ischemic pattern (infarction or myocardial edema in a coronary territory) in 53% of patients presenting with MINOCA.50 Altogether, multimodality imaging, in this case OCT combined with CMR, identified the cause of MINOCA in a higher proportion of women than OCT or CMR alone (84.5% vs. 46.2%, p<0.001 and vs. 54.1%, p=0.001, respectively).50
There are currently no imaging techniques for direct morphologic visualization of the coronary microcirculation. Therefore, evaluation relies on functional assessment of the microcirculation through non-invasive (CMR or positron emission tomography) and invasive techniques, with the latter being the standard for the diagnosis of CMD.51
Endothelium-dependent dysfunction of the microcirculation can be assessed using intracoronary acetylcholine infusion.51 In normal endothelium, acetylcholine induces vasodilatation, however, in dysfunctional endothelium this infusion triggers paradoxical arteriolar vasoconstriction.51 Intracoronary provocative tests (using acetylcholine or ergonovine) were found to be safe and useful to rule out coronary spasm as the cause of MINOCA, thereby identifying a higher risk subgroup of patients.32,52 In their systematic review, Pasupathy et al.4 report that more than a quarter of patients have inducible spasm, and this seems to have ethnic associations, being especially frequent among Japanese patients.4,12 The test is considered positive for epicardial spasm if there is focal or diffuse epicardial coronary lumen reduction of ≥90% in comparison to the relaxed state of the vessel, associated with reproduction of the patient's symptoms and ischemic ECG findings. It is considered positive for microvascular spasm when typical ischemic ST-segment changes and angina occur without coronary constriction of ≥90%.32 However, unlike intracoronary imaging, these procedures should not be performed in the acute phase.30
Endothelium-independent microcirculatory dysfunction can be also assessed by coronary flow reserve (CFR) and by the index of microvascular resistance (IMR).51 These are measured using intravenous vasodilators such as adenosine. CFR represents the ability of coronary blood flow to match metabolic demand and reflects the combined vasodilator capacity of epicardial and microvascular coronary arteries. IMR can be measured by thermodilution or intravascular Doppler in a hyperemic condition (induced by adenosine), and estimates the minimum achievable microvascular resistance.51,53 Values of CFR <2.0 or IMR ≥25 units indicate abnormal microvascular function.51,53
Testing for thrombophilia should also be routinely performed in MINOCA patients.54 Hypercoagulable disorders, which cause coronary thrombosis, can be divided into inherited and acquired causes.12 Inherited thrombophilia disorders can be detected in 14% of MINOCA patients,4 with some recent studies reporting a higher prevalence.54 Factor V Leiden is present in 3–7% of Western populations, however a systematic review4 found that 12% of MINOCA patients carry this mutation. Antiphospholipid syndrome is also frequent.54 The prevalence of factor V Leiden is higher in MINOCA patients than in patients with MI-CAD, suggesting that thrombophilia might play a more important role in non-obstructive MI,4 making it reasonable to consider this etiology in MINOCA patients, especially younger women.
TreatmentDespite MINOCA's high prevalence and less favorable prognosis than originally hypothesized, there is still a lack of data and consensus on diagnostic strategies, management and therapeutic interventions.13 A recent review26 focusing on the treatment of MINOCA patients reported a scarcity of high-level evidence on treatment strategies.
Specific treatment for MINOCA depends on the underlying pathophysiological mechanisms,28 but the mechanism leading to MI is not identified in many cases.
MINOCA patients are less likely to be discharged with optimized medical therapy than MI-CAD patients.9,55 In the SWEDEHEART registry, 85% of MINOCA patients were discharged on statins, 83% on beta-blockers, 66% on dual antiplatelet therapy (DAPT), and 64% on angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs).56 Paolisso et al.9 reported even lower proportions of patients discharged with medical therapy, especially DAPT (42%) and statins (64%), with high variability between hospitals.57
Since patients with MINOCA have less than 50% coronary artery obstruction, revascularization is typically not an option.12,26 For medical therapy, a large-scale multicenter trial (MINOCA-BAT)5 is currently recruiting patients discharged after MINOCA without clinical signs of heart failure and with LV ejection fraction ≥40%, with the aim of assessing whether conventional secondary prevention therapies (beta-blockers and/or ACEIs/ARBs) are beneficial in these patients (reducing the composite endpoint of all-cause death, admission for MI, ischemic stroke or heart failure). In an observational study of the large multicenter SWEDEHEART registry, Lindahl et al.56 reported that the risk of major adverse cardiac events (MACE) in patients under ACEIs/ARBs was 18% lower than in those not on ACEIs/ARBs, and 23% lower in patients treated with statins compared to their non-statin counterparts. Beta-blockers showed 14% lower risk, although this was not statistically significant. In contrast, DAPT was found to have a nonsignificant 33% higher risk of hospitalization due to a bleeding event.56
These findings conflict to some extent with other studies. Renin-angiotensin-aldosterone system inhibitors are reported as beneficial agents,9,58,59 although some studies, including a large multicenter cohort study, found no significant association with a better outcome.60,61 A study comparing ACEIs and ARBs found that while the incidence of MACE was comparable between groups, ACEIs were better at preventing recurrent MI (hazard ratio [HR]: 0.18, 95% confidence interval [CI]: 0.04–0.86; p=0.03).62 Statins are also fairly consistently found to be beneficial,58,60,63,64 but others report no change in outcome.9,61 A cohort study reports that beta-blockers significantly reduce the risk of MACE (HR: 0.49, 95% CI: 0.31–0.79, p=0.02),61 although others have found no significant associations.9,59,60 DAPT and aspirin appear not to be beneficial.9,61 The conflicting data may be due to variability in inclusion criteria and sample size.
In their scientific statement, the AHA detail treatment strategies for MINOCA patients.12 For those with type 1 MI, statins and antiplatelet therapy are strongly recommended. Aspirin is the main initial therapy for patients with plaque disruption (erosion and/or rupture), and a second antiplatelet agent may be reasonable.12 It is important to note that analysis of a large randomized multinational trial (CURRENT-OASIS 7)13 assessing the impact of double-dose versus standard-dose antiplatelet therapy in MINOCA patients (in this case, clopidogrel), concluded that there was no benefit of higher-dose therapy and that this higher dose was associated with possible excess cardiovascular death, MI or stroke. Antiplatelet therapy, especially at higher dosages, should accordingly not be prescribed routinely for MINOCA patients.13 This highlights the importance of finding the underlying cause of MINOCA, as a subgroup of patients, like those with type 1 MI MINOCA, might benefit from more aggressive antithrombotic therapy, but in others it may be harmful. Beta-blockers and ACEIs/ARBs should also be considered, especially in the presence of LV dysfunction.12
Calcium channel blockers are recommended as the mainstay therapy for patients with epicardial coronary vasospasm, as they have documented benefits in vasospastic angina patients.12 In patients with refractory vasospastic angina, two calcium channel blockers (operating via different receptors) can be used.12 Short-acting sublingual and intracoronary nitrates are useful in acute situations, but the benefits of long-acting nitrates are not well established.12 Other agents shown to alleviate coronary spasm include nicorandil and cilostazol.12 Statins should be considered,12 due to their improvement of endothelial function.65
For patients with coronary microvascular dysfunction, calcium channel blockers and beta-blockers are beneficial in alleviating symptoms, with nitrates showing less effect.12 Other unconventional therapies (such as l-arginine, dipyridamole or imipramine) are thought to improve endothelial function, promote microvascular vasodilation or act on other beneficial pathways, but further trials are required.12
Antiplatelet or anticoagulant therapy should be implemented in patients with evidence of coronary thrombosis/embolism, although the use of lifelong anticoagulant or antiplatelet therapy is still up for debate.12 Targeted therapies for specific hypercoagulable states should also be considered.12
SCAD commonly heals spontaneously, therefore percutaneous coronary intervention or stenting should be avoided due to the risk of further propagation of the dissection, unless the patient is unstable, with ongoing ischemia or with full coronary occlusion and STEMI. Data suggest a better outcome in these patients when treated with beta-blockers, and aspirin is also typically used. Use of DAPT and anticoagulant therapy remains controversial,66 although clopidogrel may be considered. Other cardioprotective therapies should be implemented based on the patient's risk factors and LV function. Avoiding strenuous exercise and future pregnancies are sometimes recommended, although this lacks long-term evidence-based data.12
Other supply-demand mismatch mechanisms leading to MINOCA should be treated by reversing the inciting cause.12
Exercise-based cardiac rehabilitation (CR) is the third most cost-effective intervention to reduce cardiovascular mortality, just behind aspirin and beta-blockers.60 A single-center randomized trial60 established three-year exercise-based CR as an independent protective factor for all-cause mortality and MACE in the MINOCA population (HR: 0.48, 95 CI: 0.28–0.82; p<0.01; and HR: 0.57, 95% CI: 0.40–0.83; p<0.01, respectively). Stress seems to play a role in triggering SCAD events, and therefore stress reduction may also be beneficial in these patients.67
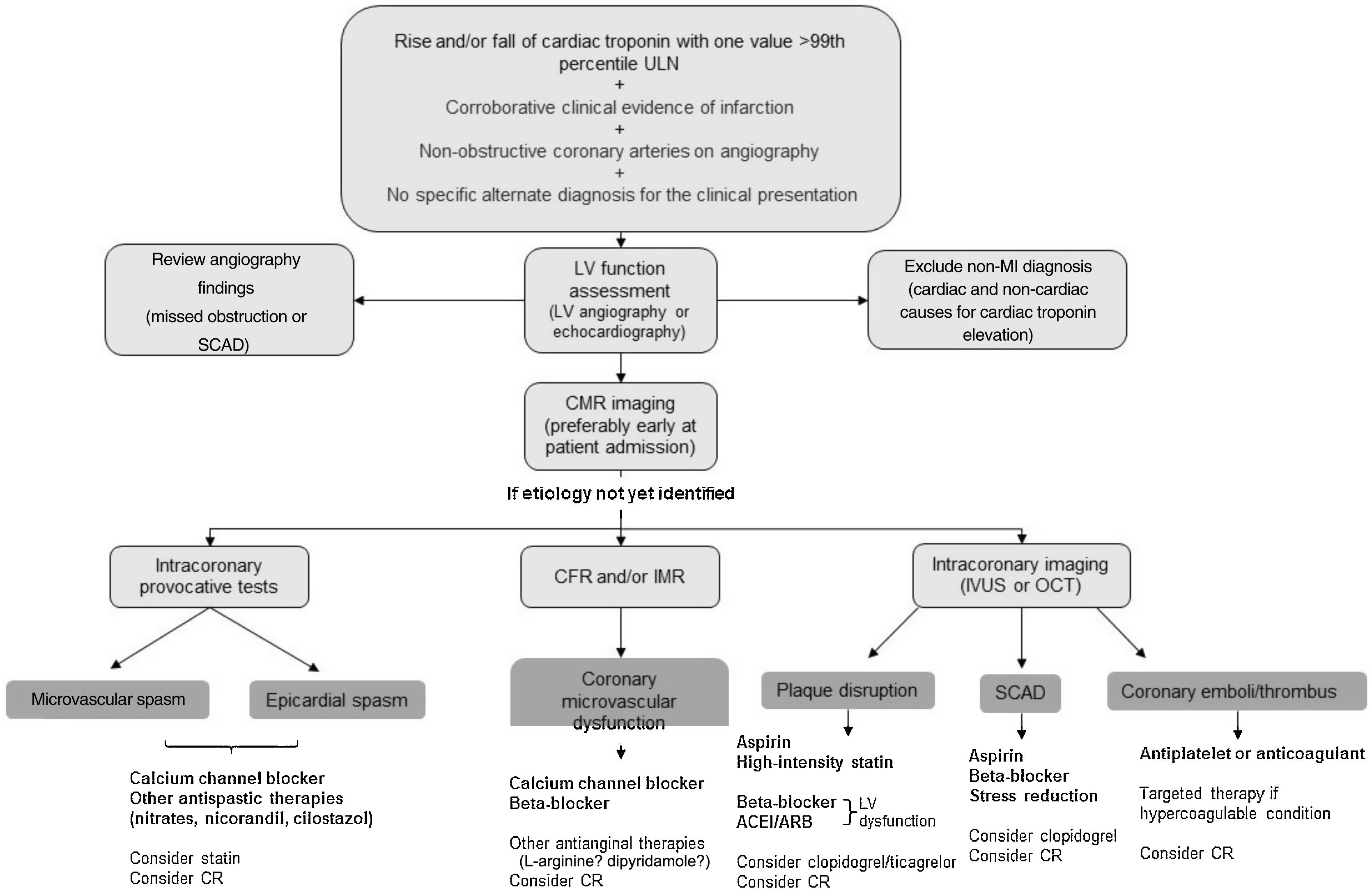
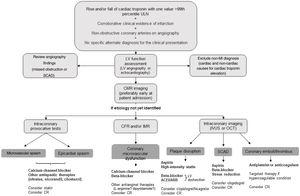
A summary of our proposed management for these patients is illustrated in Figure 2.
Proposed diagnostic algorithm and cause-targeted treatment strategies for patients with myocardial infarction with non-obstructive coronary arteries (MINOCA). ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; CFR: coronary flow reserve; CMR: cardiac magnetic resonance; CR: cardiac rehabilitation; IMR: index of microvascular resistance; IVUS: intravascular ultrasound; LV: left ventricular; MI: myocardial infarction; OCT: optical coherence tomography; SCAD: spontaneous coronary artery dissection; ULN: upper limit of normal.
The prognosis of MINOCA is variable and related to the underlying cause.68 Data on patient outcomes are somewhat conflicting.
A systematic review4 showed that compared to patients with MI-CAD, MINOCA patients had lower in-hospital mortality (1% vs. 3%) and all-cause mortality at 12 months (5% vs. 7%). Other studies and guidelines support this favorable outcome compared to MI-CAD.8,10,11,13,15,18,19,69–71 Moreover, a more recent systematic review,18 including 44 studies comprising 36932 patients, reports an even lower annual mortality of 2%, which is in line with other large-scale trials and multicenter studies. Although this might seem optimistic, the annual all-cause mortality for patients with chest pain and minor luminal irregularities is 0.3%,19,72 so it is clear that MINOCA patients represent a specific subset of patients with a more dire prognosis. Therefore, even though mortality is lower in MINOCA patients than in obstructive CAD, it remains of great concern given the limited clinical attention often received by these patients.4 García-Blas et al.71 also reported that patients with completely normal coronary arteries on angiography had lower MACE than those with mild irregularities (<30% stenosis) and moderate atherosclerosis (30–49% stenosis). This supports the idea that there are different patient groups within the MINOCA umbrella and that they need to be addressed and managed accordingly.
An observational study based on the SWEDEHEART registry, conducted over a span of 10 years with over 9000 MINOCA patients,19 identified clinical predictors of adverse outcome, such as older age, diabetes, hypertension and smoking (p<0.01). These predictors are strikingly consistent with risk factors linked with CAD.19 Nonetheless, Eggers et al.11 analyzed the same registry and compared MINOCA patients, MI-CAD patients and a control non-MI group. The MINOCA group had more major adverse events (MAE) than the control group (22% vs. 7%, p<0.01) but lower MAE than the MI-CAD group (22% vs. 26%, p<0.01).11
However, results from the VIRGO study showed that one-month and 12-month mortality for MINOCA and MI-CAD patients were 1.1% vs. 0.6% (p=0.43) and 1.7% vs. 2.3% (p=0.68), respectively.22 Although none of these differences were statistically significant, they imply that the prognosis for MINOCA may be just as somber as for traditional MI-CAD. Furthermore, psychosocial and functional status were similar in the two groups.22 In agreement, in 2021 Schmitz et al. reported similar 30-day and one-year mortality in both groups (7% vs. 8%, p=0.70 and 10% vs. 8%, p=0.55, respectively).55 Other studies report similar findings.16,58 The conflicting results from the VIRGO study, with almost 3000 patients enrolled, may be explained by population differences from other studies, such as younger age and a 2:1 female-male ratio, but mostly by the exclusion of myocarditis and TTS, unlike earlier studies. These are two very common conditions among MINOCA patients (according to earlier interpretations of the term); these patients are usually healthier than coronary ischemic patients,22 and therefore prognosis in MINOCA patients appears to be comparable to that of MI-CAD patients when these more benign pathologies are ruled out. Indeed, in the ITAMY study, a CMR study of patients with myocarditis with preserved ejection fraction, the occurrence of sudden cardiac death, appropriate implantable cardioverter-defibrillator shock and resuscitated cardiac arrest was only around 2% during a median follow-up of 1572 days.73
Compared to MI-CAD, a history of heart failure, history of chronic obstructive pulmonary disease, and male gender seem to be stronger indicators of adverse outcome in the MINOCA population.11 Older age, higher creatinine concentration, low LV ejection fraction and ST elevation have also been linked to increased mortality. Nordenskjöld et al.19 found that, among other predictors, higher C-reactive protein levels were predictive of more adverse outcomes, and recent data suggest that patients with inflammatory ruptures are at higher risk of recurrent events.68 Another study supports this association.74 A higher neutrophil-to-lymphocyte ratio at admission was associated with increased mortality, further confirming that inflammation plays a role in these patients.75
The GRACE risk score, used to predict MACE in NSTEMI patients, showed moderate predictive value when used in MINOCA patients without ST elevation (area under the curve: 0.71, 95% CI: 0.63–0.80, p<0.01), providing potentially valuable information for this subgroup of MINOCA patients.76 IMR was found to be a strong predictor of outcome in the MINOCA population and is an objective risk stratification tool: as the index rises, the risk of MACE increases.77 High-sensitivity cardiac troponin T was also confirmed as a strong independent predictor of all-cause and cardiovascular mortality, as well as MACE,78 however the same was not true for high-sensitivity troponin I in another multivariate analysis.79 Patients with positive provocative tests using ergonovine or acetylcholine constitute a high-risk subgroup,32 and reduction or suspension of calcium antagonist therapy is correlated with mortality, highlighting the role epicardial spasm plays in the onset of fatal events.32,68 The ACEF (age, creatinine, and ejection fraction) score has also been shown to have good predictive value for MACE in the MINOCA population.80
Low triiodothyronine syndrome and higher levels of lipoprotein(a) were also shown to be a predictor of worse outcomes in MINOCA.81–83 Obstructive sleep apnea-hypopnea was established as an independent predictor of all-cause mortality (HR: 1.71, 95% CI: 1.29–2.42, p<0.01) and major adverse cardiac and cerebral events (HR: 1.73, 95% CI: 1.20–2.39, p<0.01).84
Diagnostic tools can also have a role in predicting mortality. ST-segment elevation on the presentation ECG was shown to be an independent predictor of mortality in these patients.85 A study by Dastidar et al.85 that included 388 patients with MINOCA undergoing CMR assessment found that CMR was able to identify the etiology in almost three-quarters of patients (25% myocarditis, 25% MI, and 25% cardiomyopathy); the cardiomyopathy group had the worst prognosis and in a multivariate analysis, CMR diagnosis of cardiomyopathy and ST-segment elevation on presentation ECG remained the only two significant predictors of mortality.85
A systematic review found that reduced ejection fraction, use of beta-blockers during follow-up (patients with reduced ejection fraction have a class I indication for the use of beta-blockers, which might explain this association with worse outcome), malignancy, and ST-segment depression at admission are predictors of poor outcome in these patients.18 Finally, patients with mild CAD affecting three vessels or the left main stem were found to have worse long-term outcomes.74
Quality of lifeA study assessing the short-term impact of MINOCA on physical and mental status revealed that MINOCA patients had lower physical and mental scores than healthy controls and similar scores to MI-CAD patients.25 This underscores the importance of recognizing MINOCA patients as a vulnerable group of individuals who need personalized clinical care and support.
ConclusionsMINOCA is an increasingly recognized clinical entity that results from a broad range of disease mechanisms, ultimately leading to infarction. Even though no significant coronary stenosis can be documented in these patients, they are at a high risk of adverse cardiovascular events. After the exclusion of other causes for troponin elevation, identifying the underlying disease mechanism is crucial, since treatment and prognosis are closely related to the pathogenesis of the disease.
MINOCA is increasingly recognized in the European and American guidelines, and there are now clear recommendations for advanced imaging. Multimodality imaging is of utmost importance since it has proven value in diagnosing the underlying cause of MINOCA.
Overall, statins seem to be consistently documented as beneficial in these patients, as are ACEIs/ARBs, with ACEIs being the preferred option to prevent recurrent MI.62 The role of beta-blockers and antiplatelet therapy is somewhat controversial. Future clinical trials accounting for the heterogeneity of disease mechanisms in MINOCA patients are crucial to define directed therapies for each subset of MINOCA patients.
Conflicts of interestThe author has no conflicts of interest to declare.
We would like to thank Dra. Teresa Pinho and Dr. André Carvalho, from Cardiology and Radiology departments of Centro Hospitalar e Universitário São João, respectively, for the iconographic support.