Bosentan is recommended for symptomatic patients with Eisenmenger syndrome due to simple congenital lesions such as atrial and ventricular septal defects (VSD). However, its long-term efficacy and safety in patients with pulmonary arterial hypertension (PAH) associated with complex congenital heart disease (CHD) is unknown.
ObjectivesWe examined the short- and long-term effects and safety profile of bosentan in patients with PAH and complex CHD.
MethodsWe followed 14 patients with PAH and complex CHD for a mean of four years. Demographic parameters, exercise capacity assessed by the six-minute walking test (6MWT) and oxygen saturation were assessed at baseline, six months and at follow-up.
ResultsMean age was 37.1±11.7 years; 90% were in WHO class III or IV. The most common diagnosis was pulmonary atresia with VSD (35.7%), followed by truncus arteriosus (28.6%), patent ductus arteriosus (21.4%) and transposition of the great arteries (14.3%). After six months of treatment, six-minute walking distance (6MWD) increased from 371.9 to 428.4 m (p=0.005) and functional class was improved (p=0.005). After four years, one patient discontinued bosentan due to side effects and four patients were started on sildenafil, after a mean 38 months of bosentan treatment. Mean 6MWD for patients on bosentan monotherapy (n=8) was 440.1±103.8 m, whereas for patients on bosentan-sildenafil combination therapy (n=4) it was 428.8±96.9 m, after four years of therapy. Two patients died during follow-up.
ConclusionsBosentan was safe and was associated with improved exercise capacity in patients with PAH and complex CHD. This improvement was sustained for up to four years and the safety profile was similar to simple CHD patients.
O bosentano é recomendado em doentes com hipertensão arterial pulmonar (HAP) associada a lesões congénitas simples, como comunicações interventriculares (CIV). Contudo, a sua eficácia e segurança a longo prazo em doentes com HAP associada a cardiopatias congénitas complexas (HAP-CCC) é desconhecida.
ObjectivosAvaliámos a eficácia e segurança a curto e longo-prazo do bosentano na HAP-CCC.
MétodosEstudaram-se 14 doentes com HAP-CCC, relativamente a parâmetros demográficos, capacidade funcional avaliada pelo teste de marcha de seis minutos (TM6M) e saturação de oxigénio, antes de iniciar terapêutica, aos seis meses e durante o período de seguimento clínico a longo-prazo (quatro anos).
ResultadosNoventa por cento dos doentes encontravam-se em classe OMS III ou IV, com idade média de 37,1 ± 11,7 anos. O diagnóstico mais frequente foi a atrésia pulmonar com CIV (35,7%), seguida de truncus arteriosus (28,6%), canal arterial patente (21,4%) e transposição de grandes vasos (14,3%). Após seis meses de tratamento, o TM6M aumentou de 371,9 para 428,4 metros (p = 0,005) e a classe funcional melhorou (p = 0,005). Após quatro anos, um doente suspendeu bosentano devido a efeitos secundários e quatro doentes iniciaram sildenafil, após uma duração média de monoterapia de 38 meses. Após quatro anos de terapêutica, nos doentes em monoterapia com bosentano (n = 8) o TM6M foi de 440,1 ± 103,8 metros, enquanto que nos doentes com bosentano-sildenafil (n = 4) foi de 428,8 ± 96,9 metros. Dois doentes faleceram durante o período de seguimento clínico.
ConclusõesO bosentano foi seguro e esteve associado a melhoria na capacidade funcional em doentes com HAP-CCC até aos quatro anos de seguimento clínico.
Eisenmenger syndrome (ES) is defined as significant pulmonary arterial hypertension (PAH) in conjunction with congenital heart disease (CHD) and shunt reversal,1 and comprises about 1% of all CHD.2 Although caused by a defect in the heart, ES is a multiorgan disorder associated with numerous life-threatening complications, including hemoptysis, cerebrovascular accidents, brain abscesses, arrhythmias and syncope.3 The majority of patients who develop ES survive to adulthood, but markedly diminished exercise tolerance with reduced life expectancy is the rule.4 Although their survival is better than in idiopathic pulmonary arterial hypertension (IPAH), ES patients share many histopathological similarities with IPAH.5
These similarities made the use of pulmonary vasodilators in this subset of patients an attractive option. In the BREATHE-5 study, the first placebo-controlled trial in patients with ES, the endothelin-receptor antagonist (ETRA) bosentan was well tolerated and improved exercise capacity and hemodynamics without compromising peripheral oxygen saturation,6 and these results were further supported by the open-label extension of this pivotal trial.7 However, BREATHE-5 only included ES patients with simple CHD (atrial septal defect, ventricular septal defect or both), excluding patients with complex CHD, patent ductus arteriosus or segmental pulmonary hypertension as in pulmonary atresia. Evidence for the use of bosentan in this patient subset is only available in the form of series with small samples or short follow-up.8–10 Moreover, due to the progressive nature of the disease,6 some patients may still have a negative evolution even though treated with advanced therapies, highlighting the need for combination therapy. In the only randomized controlled trial on combination therapy in ES, the addition of sildenafil to ongoing bosentan failed to improve walking distance or pulmonary vascular resistance.11
The aims of our study were (i) to assess the safety of bosentan and its short-term effects on systemic oxygen saturation and exercise capacity in patients with complex CHD and PAH, and (ii) to perform a long-term follow-up to assess efficacy and safety of bosentan treatment and the need for combination therapy in this group of complex CHD patients.
MethodsParticipantsWe prospectively collected data on a dedicated database of all patients with PAH due to complex CHD, who were being followed or were referred to our tertiary center between August 2003 and July 2009. All patients had complex CHD, with Eisenmenger physiology (non-restrictive intra- or extracardiac communication with a right-to-left shunt at rest and high pulmonary vascular resistance) or with severe PAH. Right heart catheterization was performed in all patients to confirm the diagnosis before treatment initiation. Patients with isolated atrial or ventricular septal defects were excluded from the analysis. Functional class was II–IV in all participants, assessed by the World Health Organization (WHO) classification. All pulmonary vasodilator prescriptions were authorized by the pharmacy and this investigation conformed to the principles outlined in the Declaration of Helsinki. The local ethics committee approved the research protocol and informed consent was obtained from all subjects. Data up to May 2011 were analyzed.
MedicationBosentan treatment was initiated at a dose of 62.5 mg bid. After one month, if no adverse effects occurred, the dosage was doubled to 125 mg bid and maintained thereafter. Baseline and monthly hemoglobin and liver function tests were obtained. Treatment with prostanoids, phosphodiesterase V inhibitors and other endothelin receptor antagonists was not allowed before the introduction of bosentan. Based on patients’ overall clinical assessment, the team of attending physicians (including a cardiologist with specialization in adult congenital heart disease [GC] and a pediatric cardiologist [AMS]) was authorized to add sildenafil to the ongoing therapy in a starting dosage of 20 mg tid up to 50 mg tid. All patients had a baseline iron status assessment and bosentan therapy was initiated only after correction of anemia and/or iron deficiency.
Follow-up and final assessmentClinical follow-up was performed by a monthly clinical visit to our hospital in the first six months of treatment and in three-month periods thereafter.
Six-minute walking testSubmaximal exercise capacity was assessed by the six-minute walking test (6MWT). This test was conducted by three experienced operators in a 30-m indoor marked corridor, according to the guidelines of the American Thoracic Society.12 Heart rate, pulse oximetry and Borg dyspnea score were recorded at baseline and six minutes in all tests.
Statistical analysisContinuous variables are expressed as means ± standard deviation and categorical variables by percentages. Pairwise comparison between baseline and follow-up continuous variables was performed using the paired Student's t test. Due to the small sample size, all variables were tested for normality using the Kolmogorov-Smirnov test. Categorical variables at baseline and follow-up were compared using Fisher's test. All p-values were two-sided and a p-value less than 0.05 was considered to indicate statistical significance. Analyses and calculations were performed using SPSS version 13.0 (Chicago, USA).
ResultsBaseline demographics and characteristicsPatients’ baseline characteristics are summarized in Table 1. Fourteen patients were enrolled in the treatment protocol. Mean age was 37.1±11.7 years and half of the patients were female. The most common diagnosis was pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries (35.7%) (one patient had a palliative Blalock-Taussig shunt), followed by truncus arteriosus with ventricular septal defect (28.6%), patent ductus arteriosus with ventricular septal defect (21.4%) and transposition of the great arteries (14.3%) (one patient underwent a Mustard procedure). More than half of the patients were taking diuretics (57.1%) and six had an oxygen prescription; no patient was taking warfarin. At baseline, all patients were normotensive (mean 114±17/71±24 mmHg), with heart rates in the high normal range (mean 88±12 bpm). Baseline hemoglobin was elevated (17.9±2.1 g/dl); all patients had normal liver function tests. Hemodynamic findings were remarkable for a very high mean pulmonary artery systolic pressure (79.6±31.3 mmHg). Regarding functional capacity, more than 90% of patients were in WHO class III or IV. Only one patient was in WHO class II before starting bosentan. No phlebotomies were conducted in the cohort.
Baseline characteristics.
| Clinical characteristics | n=14 |
| Age, years | 37.1±11.7 |
| Female gender, n (%) | 7 (50) |
| Diagnosis | |
| Truncus arteriosus, n (%) | 4 (28.6) |
| Transposition of the great arteries, n (%) | 2 (14.3) |
| Pulmonary atresia with ventricular septal defect, n (%) | 5 (35.7) |
| Patent ductus arteriosus, n (%) | 3 (21.4) |
| Medications | |
| Warfarin, n (%) | 0 (0.0) |
| Digoxin, n (%) | 3 (21.4) |
| Diuretics, n (%) | 8 (57.1) |
| Mean daily furosemide dosage (mg) | 37.5 |
| Beta-blockers, n (%) | 1 (7.1) |
| Oxygen, n (%) | 6 (42.9) |
| Laboratory variables | |
| Hemoglobin, g/dl | 17.9±2.1 |
| Mean corpuscular volume, fl | 89.0±5.4 |
| Creatinine, mg g/dl | 0.8±0.4 |
| Alanine aminotransferase, U/l | 18.4±4.7 |
| Hemodynamic variables | |
| Mean pulmonary artery pressure, mmHg | 79.6±31.3 |
Median exposure to bosentan was 43 (interquartile range 30-67) months. One patient discontinued bosentan after 16 months of usage due to low treatment adherence secondary to lower limb edema and was started on sildenafil. During long-term follow-up, four patients were started on sildenafil and one on triple combination therapy with intravenous iloprost due to progressive heart failure. Sildenafil was added after a median bosentan treatment duration of 38 months. Patients who needed combination therapy had a lower six-month 6MWT response after bosentan initiation (+22 m) compared to patients who did not need therapeutic escalation during long-term follow-up (+85 m). Table 4 summarizes the data for each patient.
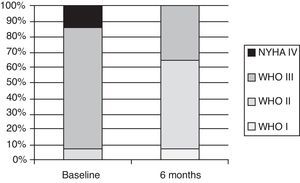
Exercise capacity and World Health Organization functional classAfter six months of bosentan treatment, 6MWD increased in 12 and decreased two patients. The mean increase in 6MWD was 56.5 m, from 371.9±90.3 to 428.4±98.3 m (p=0.005) (Table 2). Changes in functional class were significant, as shown in Figure 1. After a median follow-up of four years, mean 6MWD for patients on bosentan monotherapy (n=8) was 440.1±103.8 m, whereas for patients on bosentan-sildenafil combination therapy (n=4) it was 428.8±96.9 m (Table 3). Regarding functional class, after a mean six months of treatment with bosentan, nine patients were in class I or II and five patients remained in class III (p=0.005). No patient experienced worsening in functional class.
Clinical characteristics at baseline and six months after bosentan therapy.
| Parameter | Baselinen=14 | 6 monthsn=14 | Mean difference (95% CI) | p |
| Heart rate, beats/min | 88±12 | 90±14 | 2 (−5-9) | 0.765 |
| Systolic blood pressure, mmHg | 114±17 | 119±12 | 5 (−3-13) | 0.170 |
| Diastolic blood pressure, mmHg | 71±24 | 59±12 | −12 (−28-4) | 0.125 |
| WHO class | ||||
| I, n (%) | 0 (0.0) | 1 (7.1) | ||
| II, n (%) | 1 (7.1) | 8 (57.1) | ||
| III, n (%) | 11 (78.6) | 5 (35.7) | ||
| IV, n (%) | 2 (14.3) | 0 (0.0) | 0.895 | |
| Six-minute walking distance, m | 371.9±90.3 | 428.4±98.3 | 56.5 (14.3-98.6) | 0.005 |
| Systemic O2Sat at rest, % | 82.0±6.9 | 81.9±6.6 | −0.1 (−2.7-2.8) | 0.956 |
| Systemic O2Sat at peak exercise, % | 73.4±13.2 | 72.8±10.6 | −0.6 (−5.1-6.3) | 0.832 |
| Peak SBP, mmHg | 123±16 | 130±13 | 7.0 (−0.3-14.3) | 0.058 |
| Borg dyspnea score, baseline | 2.4±1.7 | 3.3±2.3 | 0.9 (−0.8-2.6) | 0.271 |
| Borg dyspnea score after exercise | 6.7±1.8 | 7.5±1.5 | 0.8 (−0.2-1.8) | 0.108 |
O2 Sat: oxygen saturation; SBP: systolic blood pressure. WHO: World Health Organization functional class.
Individual data for patients that required therapeutic escalation.
| DOB | Gender | Vital status | Diagnosis | WHO initial | 6MWT initial | Bosentan start date | WHO6M | 6MWT6M | Sildenafil start date | 6MWT prior | 6MWD6M | WHO6M | Date of death | Cause of death |
| 13 Jan 63 | F | Alive | PDA and VSD | III | 258 | Jun 04 | III | 218 | Aug 08 | 370 | 392 | III | ||
| 6 Jun 70 | F | Alive | TGA | III | 517 | Jan 07 | II | 550 | Feb 10 | 450 | 540 | III | ||
| 29 Aug 93 | M | Alive | PDA and VSD | III | 441 | Aug 03 | II | 490 | Feb 10 | 410 | 468 | II | ||
| 22 Jul 61 | M | Deceased | TA | IV | 324 | Aug 05 | II | 370 | Apr 08 | 180 | 200 | IV | Aug 09 | RHF |
DOB: date of birth; F: female; M: male; PDA: patent ductus arteriosus; RHF: right heart failure; 6M: six-month; 6MWD: six-minute walking distance, in m; TA: truncus arteriosus; TGA: transposition of the great arteries; VSD: ventricular septal defect; WHO: World Health Organization functional class.
Results in patients with segmental pulmonary hypertension due to pulmonary atresia (n=5) were similar to those of the overall population. After six months, mean 6MWD improved from 352.6±93.2 m to 454.0±72.7 m (p=0.043). The improvement in functional capacity was maintained at four years (451.8±54.7 m, p=0.043 vs. baseline and p=0.933 vs. six months). No differences were found regarding oxygen saturation pre- and post-exercise; no patient required therapeutic escalation.
Safety, morbidity and mortalityOne patient required discontinuation of bosentan due to lower limb edema. No significant rise in liver transaminases was seen during follow-up and none of the patients had levels exceeding three times the upper limit of the reference range. No signs of decreased oxygen saturation were seen.
Two patients (14.3%), both with truncus arteriosus, died during follow-up after 48 months of vasodilator therapy. One patient (age 45) on bosentan monotherapy died due to a complicated cerebral abscess. The second patient (age 48), on triple combination therapy, died of decompensated heart failure. Survival rates in patients with complex CHD at one, two and four years were 100%, 100% and 85.7%, respectively.
DiscussionIn patients with PAH and complex CHD, bosentan was a safe, well tolerated and effective therapy. The effects on 6MWT were evident after six months and were sustained in long-term follow-up. The safety and drug tolerance were similar to those reported in the IPAH and BREATHE-5 studies, with no serious side effects occurring during this long follow-up period.
Patients with PAH and complex CHD have a better long-term prognosis than those with IPAH,13 mainly due to preservation of biventricular function until the later stages of disease. Some authors suggest that the right ventricle is ‘primed’ for a lifetime of functioning at systemic level pressure, with persistence of a protective fetal morphology.14 Unfortunately, and in contrast to previous claims that the hemodynamics of patients with ES was stable over time, the BREATHE-5 study demonstrated that even in a limited 16-week follow-up, indicators of progressive vascular disease, such as pulmonary vascular resistance index, worsened in the placebo group.6
The available evidence regarding the use of disease-targeting therapies has recently been reviewed elsewhere.15 The pivotal role of endothelin-1 in the development of pulmonary vasculopathy made the use of ETRA attractive and several studies, including one randomized controlled trial, established the benefit of ETRA in ES patients with simple defects.3,5 The evidence for sildenafil or prostacyclins is based on smaller studies.9 However, BREATHE-5 excluded patients with complex CHD and a recent single-center, randomized placebo-controlled study with 21 patients had only one patient with complex disease. Although it was thought that the location of the shunt (pre-tricuspid versus post-tricuspid) would influence treatment outcomes,16 a subgroup analysis of the BREATHE-5 study and two recent reports do not support this hypothesis.9,17 Moreover, a comparison of patients with persistent PAH after shunt closure and ES patients found no differences in four-year survival.17
After the use of bosentan, we noted a significant improvement in exercise capacity and in WHO functional class, with no decrease in Borg dyspnea score, indicating that these patients increased their exercise capacity with a similar level of perceived dyspnea. Moreover, mean six-month improvement in 6MWD was 56 m, similar to other reports, including patients with non-complex disease15 and with IPAH.5 Our results reproduce, in a larger population, the findings of Diaz-Caraballo et al., the only study to date focusing solely on complex congenital heart disease. In that study, mean 6MWD improved significantly at six months (from 266±161 to 343±121 m, p=0.045), the change being maintained at two years.8 We extend these findings to a four-year follow-up.
We also included pulmonary atresia patients, a distinct subgroup, as they present with segmental pulmonary hypertension and no shunt reversal. Little evidence is available regarding the role of pulmonary vasodilators in these patients. A recent report by Schuuring et al. on seven patients demonstrates a 62-m improvement in 6MWD after one year of bosentan treatment.10 Our results are similar, with a significant improvement in walking distance at six months, sustained in the long term. Interestingly, none of the patients needed combination therapy. The mechanism whereby bosentan acts on this subset of patients is not known, but may be related to its action on the endothelin pathway.
Although not evidence-based but in line with recommendations for IPAH,5 combination therapy with sildenafil was initiated in four patients due to disease progression, reflecting the high-risk group that was studied and the progressive nature of the disease during long-term follow-up. It is noteworthy that the four patients who underwent therapeutic escalation remained clinically stable for almost four years before the initiation of sildenafil.
Patients with PAH and complex CHD are at high risk of death18 but there is a paucity of data regarding survival in such patients under vasodilator therapy. In our cohort, total mortality was 14% at four years, which is higher than in the series by Dimopoulos et al.19 (around 3% at two years), lower than that reported by Vis et al.17 (20% at four years), but similar to the recently published results by Monfredi et al.20 Differences in study design, follow-up duration and population selection may be responsible for these disparities.
The safety of bosentan in this high-risk population was remarkable. No patient experienced a rise in liver enzymes exceeding three times the upper limit of normal or systemic hypotension. Only one patient discontinued bosentan due to lower limb edema. Moreover, and in line with previous reports, no changes were noted in systemic oxygenation. This may be due to the limited influence of bosentan on the net right-to-left shunt because its effects on both pulmonary and systemic vascular resistance are similar.6
This was a non-randomized, single-center study, with a limited number of patients reflecting the rarity of this condition. It represents a cohort of patients being followed in a specialized pulmonary hypertension clinic. Our small sample precludes further comparisons between different types of congenital defects. Although only patients with right heart catheterization-confirmed pulmonary hypertension were included in this study, the heterogeneity of the congenital lesions causing it may have influenced clinical evolution, themselves affecting the outcome. An example is truncal valve regurgitation in truncus arteriosus patients; both patients who died had severe regurgitation that may have contributed to their dismal prognosis. Several factors can influence exercise capacity in ES patients, including iron status,21 associated cardiac lesions or pulmonary comorbidities; however, our results regarding exercise capacity and functional status were consistent with other studies in patients without complex congenital disease, suggesting that bosentan has a true therapeutic effect; all patients had regular iron status assessments and correction as needed. We did not perform hemodynamic re-evaluation of patients after pulmonary vasodilator therapy; all clinical decisions regarding therapeutic escalation were made in the light of clinical, laboratorial, echocardiographic and functional data.
ConclusionsBosentan was safe and was associated with improved exercise capacity in patients with complex congenital heart disease and PAH. The improvement in exercise capacity was maintained up to four years and the longer-term safety profile was similar to that reported in simple congenital heart disease patients.
Conflicts of interestGraça Castro and António Marinho da Silva are consultants for Actelion Portugal. Rui Baptista has received funding for research from Actelion Portugal. Pedro Monteiro and Luís Augusto Providência have no relationships relevant to the contents of this paper to disclose.
We thank our nurses, Esmeralda Carvalho, Martiza Ribeiro and Sandra Mendes, for data collection.