The SANTORINI study is the first large-scale, European observational study conducted following the release of the 2019 European Society of Cardiology/European Atherosclerosis Society ESC/EAS guidelines on dyslipidemia management. This analysis aims to assess lipid-lowering therapy (LLT) use and low-density lipoprotein cholesterol (LDL-C) goal attainment in patients at high or very high cardiovascular (CV) risk enrolled in Portugal.
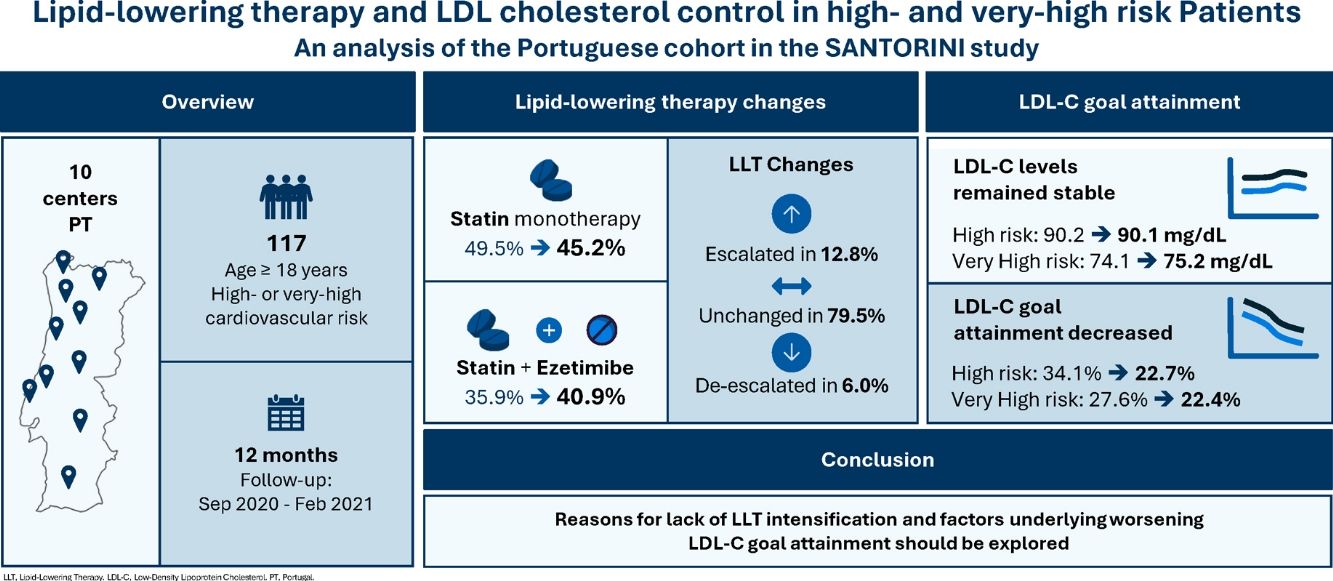
MethodsIn Portugal, 117 patients were enrolled across 10 sites between September 2020 and February 2021. Paired LDL-C values at baseline and one-year follow-up were available for 102 patients. LDL-C levels, LLT utilization patterns, and LDL-C goal attainment (as per the 2019 ESC/EAS guidelines) were assessed at both time points and compared with the broader European cohort, excluding Portuguese participants.
ResultsOver the one-year follow-up, the use of statin monotherapy decreased from 49.5% to 45.2%, while combination therapy with statin and ezetimibe increased from 35.9% to 40.9%. LLT intensity was escalated in 12.8% of patients, unchanged in 79.5%, and de-escalated in 6.0%. Mean LDL-C levels were similar between baseline and one-year follow-up: corresponding values were 90.2 mg/dL and 90.1 mg/dL in high-risk patients, and 74.1 mg/dL and 75.2 mg/dL in very high-risk patients. LDL-C goal attainment declined from 34.1% to 22.7% in high-risk patients and 27.6% to 22.4% in very high-risk patients.
ConclusionsThe Portuguese cohort of the SANTORINI study demonstrates both encouraging developments and ongoing challenges in the real-world management of dyslipidemia following the 2019 ESC/EAS guidelines. Reasons for lack of LLT intensification and factors underlying worsening rates for LDL-C goal attainment should be explored.
O estudo SANTORINI é o primeiro estudo observacional europeu de grande escala conduzido após a publicação das diretrizes de 2019 da ESC/EAS para o controlo da dislipidemia. Esta análise tem como objetivo avaliar a utilização de terapêuticas hipolipemiantes (LLT) e a obtenção dos objetivos de colesterol de lipoproteína de baixa densidade (LDL-C) em doentes com risco cardiovascular (CV) alto ou muito alto incluídos em Portugal.
MétodosEm Portugal, foram incluídos 117 doentes em dez centros, entre setembro de 2020 e fevereiro de 2021. Estavam disponíveis valores de LDL-C no início e após um ano de seguimento para 102 doentes. Os níveis de LDL-C, os padrões de utilização de LLT e o atingimento dos objetivos de LDL-C (de acordo com as diretrizes ESC/EAS de 2019) foram avaliados em ambos os momentos e comparados com a coorte europeia, excluindo os participantes portugueses.
ResultadosDurante o ano de seguimento, a utilização de estatina em monoterapia diminuiu de 49,5% para 45,2%, enquanto a terapêutica combinada com estatina e ezetimiba aumentou de 35,9% para 40,9%. A intensidade da LLT foi intensificada em 12,8% dos doentes, manteve-se inalterada em 79,5% e foi reduzida em 6,0%. Os valores médios de LDL-C mantiveram-se semelhantes entre o início e o final do seguimento de um ano: 90,2 mg/dL e 90,1 mg/dL nos doentes de alto risco e 74,1 mg/dL e 75,2 mg/dL nos doentes de muito alto risco. A proporção de doentes que atingiu o objetivo de LDL-C diminuiu de 34,1% para 22,7% nos doentes de alto risco e de 27,6% para 22,4% nos doentes de muito alto risco.
ConclusõesA coorte portuguesa do estudo SANTORINI evidencia avanços encorajadores, mas também desafios persistentes na gestão, em mundo real, da dislipidemia à luz das diretrizes ESC/EAS de 2019. Devem ser exploradas as razões para a ausência de intensificação da LLT, bem como os fatores associados à redução na taxa de atingimento dos objetivos de LDL-C.
Cardiovascular disease (CVD) remains the leading cause of mortality worldwide, accounting for approximately 20.5 million deaths in 2021.1 Ischemic heart disease and stroke together represent the vast majority of these deaths, comprising around 85% of all CVD-related mortality.1 In Portugal, CVD is likewise the primary cause of death, responsible for 26.5% of all fatalities in 2022.2 That year, ischemic heart disease accounted for 5.5% of total deaths, while cerebrovascular disease contributed to 7.7%.2
Dyslipidemia is a major modifiable risk factor for atherosclerotic CVD (ASCVD), particularly in relation to myocardial infarction (MI), ischemic stroke, and peripheral arterial disease.3–5 Extensive evidence from epidemiological studies and Mendelian randomization analyses has established a causal role for low-density lipoprotein cholesterol (LDL-C) in the development of atherosclerotic plaque and the occurrence of subsequent cardiovascular (CV) events.6 Consistent findings from randomized controlled trials have demonstrated that reducing LDL-C levels safely lowers the risk of CV events.7,8 A landmark meta-analysis showed that each 1 mmol/L reduction in LDL-C with statin therapy, sustained over five years, results in a 21% relative reduction in major vascular events – regardless of sex, baseline LDL-C concentration, or history of vascular disease.9 Furthermore, LDL-C lowering with non-statin therapies – including ezetimibe, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, and bempedoic acid – has been shown to confer comparable CV risk reduction per mmol/L decrease in LDL-C, reinforcing the principle that “lower is better” for LDL-C.7,10,11
Lowering LDL-C is a cornerstone of therapy in patients at high or very high risk for ASCVD, as well as in those with established ASCVD.12 The 2019 guidelines from the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) recommend a ≥50% reduction in LDL-C from baseline, with target levels of <70 mg/dL for high-risk individuals and <55 mg/dL for those at very high risk, including patients with documented ASCVD.12 Importantly, emerging evidence highlights that the duration of LDL-C lowering is as critical – if not more so – than the absolute LDL-C level achieved or the intensity of LDL-C reduction, emphasizing the long-term benefit of sustained lipid control.13
Despite strong evidence and clear guideline recommendations, real-world data consistently show low rates of LDL-C goal attainment. The EUROpean Action on Secondary and Primary Prevention by Intervention to Reduce Events (EUROASPIRE) V survey assessed lipid control among 7824 patients with coronary heart disease (CHD) across 27 countries and found that although nearly half were receiving high-intensity lipid-lowering therapy (LLT), only 32.3% of men and 23.1% of women achieved LDL-C levels below 70 mg/dL – the recommended target for very high-risk individuals at the time of the study.14 Similarly, the EU-wiDe cross-sectionAl obserVatIoNal study of lipid-modifying therapy use in seCondary and prImary care (DA VINCI) study, a cross-sectional observational analysis of 5888 patients from 18 countries receiving LLT, which was high-intensity in 38% of patients, reported that just 33% met the 2019 ESC/EAS LDL-C targets overall, with only 18% of very high-risk patients achieving the <55 mg/dL threshold.15 The Treatment of high and very high riSk dyslipidemic pAtients for the preveNTion of cardiOvasculaR events in Europe–a multInatioNal observatIonal (SANTORINI) study, which included 9136 high- and very high-risk patients from 14 European countries, further highlighted this gap.16 At baseline, 44% of patients were on high-intensity LLT, increasing to 60% at one year.17,18 Despite treatment intensification in approximately one-third of participants, only 25.5% of patients receiving monotherapy and 39.4% of those on combination LLT reached their LDL-C targets after one year.18
The SANTORINI study is the first large-scale European observational study conducted after the release of the 2019 ESC/EAS guidelines, designed to evaluate real-world patterns in the use of LLT and its effectiveness in managing LDL-C levels in high- and very high-risk patients.16 In this analysis, we focus on the cohort of patients enrolled in Portugal.
ObjectivesThe aims of this analysis were to describe patient characteristics, assess patterns of LLT use, and evaluate the extent to which LDL-C targets – defined by the 2019 ESC/EAS dyslipidemia management guidelines – are being achieved in routine clinical practice.
MethodologyStudy designSANTORINI (NCT04271280) was a prospective, observational, and descriptive study in high and very high CV risk patients across 14 European countries. Patients were recruited from March 2020 to February 2021, followed by one year of prospective follow-up (approximately 12±2 months after baseline). The rationale and methodology used in SANTORINI have been described previously.16 The primary objective of the baseline report was to describe how physicians assessed CV risk, how they then approached LLT and to what extent the approaches resulted in attainment of the 2019 ESC/EAS LDL-C goals. The primary objective of the one-year follow-up was to assess changes in LLT and attainment of risk-based LDL-C goals (as per the 2019 ESC/EAS dyslipidemia guidelines). Baseline and one-year follow-up data were collected from patient records. As SANTORINI is a non-interventional study, no formal visits, examinations, laboratory tests or procedures were mandated. All treatment decisions were left to the discretion of the attending physician, and the study sponsor did not provide any medication.
Study participants and variablesPatients were eligible for enrollment if they required LLT, were ≥18 years of age and considered by the investigator to be at high or very high CV risk. Investigators documented the basis for the CV risk category assigned to each study participant at enrollment. CV risk category was also assessed centrally using baseline patient data and applying the criteria of the 2019 ESC/EAS guidelines. There were no specific exclusion criteria, but study participants had to have an anticipated life expectancy of more than one year. The SANTORINI study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice. All patients were asked to provide written informed consent before enrollment. Patients were recruited from different care settings (primary and secondary care) and across different specialties.
Patients’ characteristics, medical history, LLT, and other concomitant medications were documented at baseline. The LDL-C values were considered as reported by the investigators. LDL-C goal attainment was based on thresholds from the 2019 ESC/EAS guidelines: <55 mg/dL for patients at very high risk and <70 mg/dL for patients at high risk. Cardiovascular events of interest included CV death, three-component major adverse CV events (CV death, nonfatal MI, or nonfatal stroke), and four-component major adverse CV events (CV death, nonfatal MI, nonfatal stroke, or coronary revascularization). All-cause death was also assessed as an exploratory endpoint. Deaths of unknown cause were considered in the analysis of CV death. Events were not adjudicated in this observational study.
Statistical analysisThe baseline analysis dataset consisted of those patients from all documented patients (all patients with any electronic case report form) with adequate baseline information, including completing medical review of all open queries. The LDL-C dataset consisted of all study participants in the baseline analysis dataset with LDL-C data available at both baseline and follow-up. In the overall SANTORINI study, the baseline analysis dataset comprised 9136 patients, of whom 7210 patients were included in the LDL-C dataset. Analyses of baseline characteristics, LLT, and CV events of interest were implemented on the baseline analysis dataset. Analyses of LDL-C values and LDL-C goal attainment across follow-up were implemented on the LDL-C dataset. Missing LDL-C values were calculated using the Friedewald formula only if total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) values were collected at the same date.
Descriptive statistics are presented as mean (standard deviation [SD]) or median (interquartile range [IQR]) for continuous variables, and as counts and percentages for categorical variables. Missing data were not imputed, and formal statistical tests were not performed. Subgroup analyses were performed based on investigator-assessed CV risk classification at baseline and ASCVD status at baseline. Modifications in LLT intensity between baseline and 1-year follow-up were classified into three groups as previously described: no change=no change in LLT; escalation=increase in the number or intensity of LLT; de-escalation=decrease in the number or intensity of LLT.18 The incidence of CV events of interest and all-cause death during follow-up were estimated based on first events and are presented as event rates per 100 patient-years at risk. All statistical analyses were performed using Statistical Analysis System (SAS®) Version 9.4.
ResultsBaseline demographics and patient characteristicsIn Portugal, 121 patients were enrolled from September 2020 to February 2021 (Figure 1) and clean baseline data were obtained for 117 patients from 10 sites (baseline analysis dataset). One patient was excluded because enrollment was concluded before adequate baseline information had been recorded in the electronic case report form. Three patients were excluded because of incomplete medical review of all open queries. Of the 117 patients comprising the baseline analysis dataset, 71 patients (60.7%) had established ASCVD. LDL-C values were available for 112 patients at baseline. Of these 112 patients, 102 patients (91.1%) had LDL-C data available at both baseline and 1-year follow-up and were included in the LDL-C dataset. In the baseline analysis dataset, 69 patients (59.0%) were recruited at a hospital center, and the remaining 48 patients (41.0%) were recruited at a medical practice center. Most of the patients were enrolled by a cardiologist (N=65, 55.5%), followed by endocrinology (N=38, 32.5%) and internal medicine (N=14, 12.0%).
Baseline characteristics of the patients enrolled in Portugal are presented in Table 1 (baseline analysis dataset). Mean age was 63.9 years and 34 patients (29.1%) were women. Among patients reported to have ASCVD, study investigators categorized the CV risk as very high in 59 patients (83.1%) and high in the remaining 12 patients (16.9%).
Baseline demographic characteristics and CV risk factors of the participants from Portugal included in the SANTORINI study (baseline analysis dataset).
| Characteristic | Baseline analysisdataset(N=117) | CV risk classification as reported by investigator | |
|---|---|---|---|
| High risk(N=51) | Very High risk(N=66) | ||
| Female, n (%) | 34 (29.1) | 14 (27.6) | 20 (30.3) |
| Age, years, mean (SD) | 63.9 (11.4) | 63.7 (11.8) | 64.0 (11.2) |
| Hypertension, n (%) | 85 (72.7) | 36 (70.6) | 49 (74.2) |
| Diabetes, n (%) | 66 (56.4) | 37 (72.6) | 29 (43.9) |
| Familial hypercholesterolemia, n (%) | 7 (6.0) | 3 (5.9) | 4 (6.1) |
| Smoking history, n (%) | |||
| Current | 21 (18.0) | 6 (11.8) | 15 (22.7) |
| Former | 48 (41.0) | 19 (37.3) | 29 (43.9) |
| Never | 45 (38.5) | 23 (45.1) | 22 (33.3) |
| Systolic BP, mmHg, mean (SD) | 135.0 (16.6) | 134.2 (18.3) | 135.6 (15.3) |
| Diastolic BP, mmHg, mean (SD) | 75.7 (11.2) | 76.7 (11.5) | 74.9 (11.0) |
| BMI, kg/m2, mean (SD) | 27.6 (4.5) | 28.6 (4.6) | 26.8 (4.3) |
| eGFR, mL/min/1.73 m2, mean (SD) | 74.7 (29.5) | 71.8 (28.6) | 76.9 (30.2) |
| LDL-C, mg/dL | 80.8 (32.8) | 88.6 (37.1) | 74.7 (27.9) |
BMI: body mass index; BP: blood pressure; CV: cardiovascular; eGFR: estimated glomerular filtration rate; LDL-C: low-density lipoprotein cholesterol; SD: standard deviation.
The use of LLT at baseline and at one-year follow-up in the baseline analysis dataset is reported in Table 2.
Use of LLT at baseline and at 1-year follow-up by SANTORINI study participants enrolled in Portugal (baseline analysis dataset).
| LLT, n (%) | Overall(N=117) | High CV riskc(N=51) | Very high CV riskc(N=66) | |||
|---|---|---|---|---|---|---|
| Baseline | 1-Year follow-up | Baseline | 1-Year follow-up | Baseline | 1-Year follow-up | |
| Missing | 0 (0.0) | 2 (1.7)a | 0 (0.0) | 1 (2.0) | 0 (0.0) | 1 (1.5) |
| No LLT | 7 (6.0) | 3 (2.6) | 1 (2.0) | 0 (0.0) | 6 (9.1) | 3 (4.6) |
| Total monotherapy | 59 (50.4) | 53 (45.3) | 31 (60.8) | 30 (58.9) | 28 (42.4) | 23 (34.9) |
| Statin alone | 58 (49.5) | 52 (44.4) | 30 (58.8) | 29 (56.9) | 28 (42.4) | 23 (34.9) |
| Missing intensity | 5 (4.3) | 1 (0.9) | 3 (5.9) | 1 (2.0) | 2 (3.0) | 0 (0.0) |
| Low intensity | 1 (0.9) | 0 (0.0) | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate intensity | 38 (32.5) | 40 (34.2) | 21 (41.2) | 22 (43.1) | 17 (25.8) | 18 (27.3) |
| High intensity | 14 (12.0) | 11 (9.4) | 5 (9.8) | 6 (11.8) | 9 (13.6) | 5 (7.6) |
| Ezetimibe alone | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PCSK9i alone | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any other oral LLT aloneb | 1 (0.9) | 1 (0.9) | 1 (2.0) | 1 (2.0) | 0 (0.0) | 0 (0.0) |
| Total combination therapy | 51 (43.6) | 59 (50.4) | 19 (37.2) | 20 (39.2) | 32 (48.5) | 39 (59.1) |
| Statin+ezetimibe | 42 (35.9) | 47 (40.2) | 15 (29.4) | 16 (31.4) | 27 (40.9) | 31 (47.0) |
| Missing intensity statin | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.5) | 0 (0.0) |
| Low intensity statin | 1 (0.9) | 1 (0.9) | 1 (2.0) | 1 (2.0) | 0 (0.0) | 0 (0.0) |
| Moderate intensity statin | 27 (23.1) | 29 (24.8) | 11 (21.6) | 12 (23.5) | 16 (24.2) | 17 (25.8) |
| High intensity statin | 13 (11.1) | 17 (14.5) | 3 (5.9) | 3 (5.9) | 10 (15.2) | 14 (21.2) |
| PCSK9i combination | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any other combination LLTb | 9 (7.7) | 12 (10.3) | 4 (7.8) | 4 (7.8) | 5 (7.6) | 8 (12.1) |
CV: cardiovascular; LLT: lipid lowering therapy; PCSK9i: proprotein convertase subtilisin/kexin type 9 inhibitors.
At baseline, 58 patients (49.5%) were taking a statin alone, 42 patients (35.9%) were on combination LLT using a statin and ezetimibe, and nine patients (7.7%) were taking other combinations of oral LLTs. No patient was taking a PCSK9 inhibitor. Seven patients (6.0%) were not on any LLT. The dominant LLT strategy was statin monotherapy both in high CV risk patients and in very high CV risk patients. Statin intensity was more often moderate both in patients on statin monotherapy and in those taking a combination of statin and ezetimibe (see Supplementary Table 1 for statin intensity categorization).
Among the patients alive at one-year follow-up (N=115), 52 patients (45.2%) were taking a statin alone, 47 patients (40.9%) were on combination LLT using a statin and ezetimibe, and 12 patients (10.4%) were taking other combinations of oral LLTs. No patient was taking a PCSK9 inhibitor. Three patients (2.6%) were not on any LLT. The dominant LLT strategy was statin monotherapy in the high CV risk patients and a combination of a statin and ezetimibe in the very high CV risk patients. Statin intensity was more often moderate both in patients on statin monotherapy and in those taking a combination of statin and ezetimibe.
The flow of patients between different LLTs at baseline and one-year follow-up is illustrated in Figure 2. Over the course of one-year follow-up, the proportion of individuals on no LLT declined. All of the seven patients not on any LLT at baseline completed the one-year follow-up; at one-year follow-up, one of these seven patients was on a moderate intensity statin alone, four were on a combination of statin and ezetimibe (using a high-intensity statin in three and a moderate-intensity statin in one), one was on a different combination of oral LLTs, and one remained on no LLT. From baseline to one-year follow-up, the use of any LLT as monotherapy fell from 50.4% to 45.3% in the baseline analysis dataset. The reduction in statin monotherapy was small in the high CV risk group and larger in the very high CV risk group. In contrast, the use of combination LLT rose from 43.6% to 50.4%, mostly reflected by an increase in the use of a statin and ezetimibe combination. The increase in the combination of a statin and ezetimibe was small in the high CV risk group and larger in the very high CV risk group (Table 2). Overall, the use of moderate-intensity statins as part of oral combination LLT increased modestly from 23.1% to 24.8%, whereas the use of high-intensity statins increased from 11.1% to 14.5%. The latter increment was exclusively observed in the very high CV risk group.
(A) Monotherapy and combination therapy at baseline and 1-year follow-up and (B) flow of patients between different lipid-lowering therapies at baseline and 1-year follow-up. LLT: lipid-lowering therapy. Two patients died between enrolment and 1-year follow-up, hence information on LLTs was available both at baseline and 1-year follow-up for 115 patients.
The use of LLT at baseline and at one-year follow-up in the baseline analysis dataset according to ASCVD status is described in Table 3. Among patients with ASCVD, the use of statin monotherapy declined from 43.7% at baseline to 36.6% at one-year follow-up, and the use of a combination of a statin and ezetimibe increased from 39.4% to 43.7%. Moreover, the use of a high-intensity statin in combination with ezetimibe increased from baseline to one-year follow-up, but a moderate-intensity statin remained predominant in patients taking a statin alone.
Use of LLT at baseline and at 1-year follow-up by SANTORINI study participants with and without ASCVD enrolled in Portugal (baseline analysis dataset).
| LLT, n (%) | ASCVDc(N=71) | No ASCVDc(N=46) | ||
|---|---|---|---|---|
| Baseline | 1-Year follow-up | Baseline | 1-Year follow-up | |
| Missing | 0 (0.0) | 2 (2.8)a | 0 (0.0) | 0 (0.0) |
| No LLT | 6 (8.5) | 2 (2.8) | 1 (2.2) | 1 (2.2) |
| Total monotherapy | 32 (45.1) | 27 (38.0) | 27 (58.7) | 26 (56.5) |
| Statin alone | 31 (43.7) | 26 (36.6) | 27 (58.7) | 26 (56.5) |
| Missing intensity | 2 (2.8) | 0 (0.0) | 3 (6.5) | 1 (2.2) |
| Low intensity | 0 (0.0) | 0 (0.0) | 1 (2.2) | 0 (0.0) |
| Moderate intensity | 18 (25.4) | 18 (25.4) | 20 (43.5) | 22 (47.8) |
| High intensity | 11 (15.5) | 8 (11.3) | 3 (6.5) | 3 (6.5) |
| Ezetimibe alone | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PCSK9i alone | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any other oral LLT aloneb | 1 (1.4) | 1 (1.4) | 0 (0.0) | 0 (0.0) |
| Total combination therapy | 33 (46.5) | 40 (56.3) | 18 (39.1) | 19 (41.3) |
| Statin+ezetimibe | 28 (39.4) | 31 (43.7) | 14 (30.4) | 16 (34.8) |
| Missing intensity statin | 0 (0.0) | 0 (0.0) | 1 (2.2) | 0 (0.0) |
| Low intensity statin | 0 (0.0) | 0 (0.0) | 1 (2.2) | 1 (2.2) |
| Moderate intensity statin | 17 (23.9) | 17 (24.0) | 10 (21.7) | 12 (26.1) |
| High intensity statin | 11 (15.5) | 14 (19.7) | 2 (4.4) | 3 (6.5) |
| PCSK9i combination | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any other combination LLTb | 5 (7.0) | 9 (12.7) | 4 (8.7) | 3 (6.5) |
ASCVD: atherosclerotic cardiovascular disease; LLT: lipid lowering therapy; PCSK9i: proprotein convertase subtilisin/kexin type 9 inhibitors.
Modifications of LLT intensity between baseline and one-year follow-up are reported in Table 4. In the overall baseline analysis dataset, LLT intensity was escalated more often than de-escalated (12.8% vs. 6.0%) but remained unchanged for most patients (79.5%). Although some changes in LLT intensity were more common for patients at very high CV risk compared with patients at high CV risk, most very high CV risk patients had no change in LLT intensity. Escalation of LLT intensity was slightly more common for patients at very high CV risk compared with patients at high CV risk (13.6% vs. 11.8%, respectively) but de-escalation occurred even more often (9.1% for patients at very high CV risk compared with 2.0% for patients at high CV risk).
Modifications of LLT intensity from baseline to 1-year follow-up in SANTORINI study participants enrolled in Portugal (baseline analysis dataset).
| Classification | Overall(N=117) | High CV risk(N=51) | Very high CV risk(N=66) |
|---|---|---|---|
| Missing, n (%) | 2 (1.7) | 1 (2.0) | 1 (1.5) |
| No change, n (%) | 93 (79.5) | 43 (84.3) | 50 (75.8) |
| De-escalation, n (%) | 7 (6.0) | 1 (2.0) | 6 (9.1) |
| Escalation, n (%) | 15 (12.8) | 6 (11.8) | 9 (13.6) |
A detailed definition of the three categories of LLT intensity modifications is available from Ref. 18.
CV: cardiovascular; LLT: lipid lowering therapy.
LDL-C values at baseline and one-year follow-up in the overall LDL-C dataset and by investigator-classified baseline CV risk are illustrated in Figure 3. Mean (SD) LDL-C levels were similar at baseline and one-year follow-up in the overall population (Table 5). Likewise, mean (SD) LDL-C levels remained relatively unchanged from baseline to one-year follow-up in patients at high CV risk, as well as in those at very high CV risk.
LDL-C and proportion of patients enrolled in Portugal and achieving 2019 ESC/EAS guideline recommended LDL-C goal at baseline and 1-year follow-up by investigator-classified baseline CV risk (LDL-C dataset).
| Overall(N=102) | High CV risk(N=44) | Very high CV risk(N=58) | ||||
|---|---|---|---|---|---|---|
| Baseline | 1-Year follow-up | Baseline | 1-Year follow-up | Baseline | 1-Year follow-up | |
| LDL-C, mg/dL, mean (SD) | 81.0 (33.4) | 81.6 (36.1) | 90.2 (37.3) | 90.1 (36.9) | 74.1 (28.5) | 75.2 (34.4) |
| Patients at goal, n (%) [95% CI]a,b | 31 (30.4%)[21.7%–40.3%] | 23 (22.6%)[14.9%–31.9%] | 15 (34.1%)[20.5%–49.9%] | 10 (22.7%)[11.5%–37.8%] | 16 (27.6%)[16.7%–40.9%] | 13 (22.4%)[12.5%–35.3%] |
CI: confidence interval; CV: cardiovascular; EAS: European Atherosclerosis Society; ESC: European Society of Cardiology; LDL-C: low-density lipoprotein cholesterol; SD: standard deviation.
At baseline, the proportion of patients at LDL-C goal was 30.4% overall, 34.1% in the high CV risk group, and 27.6% in the very high CV risk group (Figure 4). The proportion of patients at goal at the end of 1 year declined to 22.6% in the overall LDL-C dataset, 22.7% in the high CV risk group, and 22.4% in the very high-risk group. Among patients on LLT at baseline, stratification by treatment type at one-year follow-up showed that 26.4% of patients receiving combination LLT were at LDL-C goal at follow-up compared with 16.3% receiving a single lipid-lowering drug (Figure 5).
Attainment of 2019 ESC/EAS guideline recommended risk-based LDL-C goals at baseline and 1-year follow-up among patients receiving LLT at baseline (LDL-C dataset). EAS: European Atherosclerosis Society; ESC: European Society of Cardiology; LDL-C: low-density lipoprotein cholesterol; LLT: lipid-lowering therapy.
Among patients with ASCVD, the proportion who were at LDL-C goal was 30.0% at baseline and 25.0% at one-year follow-up (Table 6). In the group of patients without ASCVD, these proportions were 31.0% and 19.1%, respectively.
LDL-C and proportion of patients enrolled in Portugal and achieving 2019 ESC/EAS guideline recommended LDL-C goal at baseline and 1-year follow-up by ASCVD status reported at baseline (LDL-C dataset).
| Overall(N=102) | ASCVD(N=60) | No ASCVD(N=42) | ||||
|---|---|---|---|---|---|---|
| Baseline | 1-Year follow-up | Baseline | 1-Year follow-up | Baseline | 1-Year follow-up | |
| LDL-C, mg/dL, mean (SD) | 81.0 (33.4) | 81.6 (36.1) | 75.8 (28.9) | 74.0 (32.9) | 88.6 (38.0) | 92.6 (37.9) |
| Patients at goal, n (%) [95% CI]a,b | 31 (30.4%)[21.7%–40.3%] | 23 (22.6%)[14.9%–31.9%] | 18 (30.0%)[18.9%–43.2%] | 15 (25.0%)[14.7%–37.9%] | 13 (31.0%)[17.6%–47.1%] | 8 (19.1%)[8.6%–34.1%] |
ASCVD: atherosclerotic cardiovascular disease; CI: confidence interval; EAS: European Atherosclerosis Society; ESC: European Society of Cardiology; LDL-C: low-density lipoprotein cholesterol; SD, standard deviation.
In the baseline analysis dataset, one patient died due to CV causes, one patient had a nonfatal MI, and one patient underwent coronary revascularization. Overall, two patients had at least one three-component major adverse events and three patients had at least one four-component major adverse events (Table 7).
Event rate per 100 patient-years for CV death, 3-component MACE, and 4-component MACE during the one-year follow-up period among patients enrolled in Portugal (baseline analysis dataset).
| Baseline investigator risk | Parameter | Number of patients with event | 100 patient-years | |
|---|---|---|---|---|
| Event rate | 95% CI | |||
| Very high risk (N=66) | CV death | 0 | 0.0 | [0.0–0.0] |
| 4-Component MACE | 2 | 2.9 | [0.0–6.9] | |
| 3-Component MACE | 1 | 1.4 | [0.0–4.2] | |
| High risk (N=51) | CV death | 1 | 1.9 | [0.0–5.6] |
| 4-Component MACE | 1 | 1.9 | [0.0–5.6] | |
| 3-Component MACE | 1 | 1.9 | [0.0–5.6] | |
| All (N=117) | CV death | 1 | 0.8 | [0.0–2.4] |
| 4-Component MACE | 3 | 2.5 | [0.0–5.2] | |
| 3-Component MACE | 2 | 1.6 | [0.0–3.9] | |
Event rate per 100 patient-years=Number of patients with the event divided by the total exposure time for all patients at risk (years) multiplied by 100. CIs are calculated using Clopper–Pearson.
CI: confidence interval; CV: cardiovascular; MACE: major adverse cardiovascular event.
Of the 9019 patients enrolled in the other 13 countries participating in the SANTORINI study, 6438 (71.4%) were categorized as very high CV risk and 2575 (28.6%) as high CV risk by study investigators (Supplementary Table 2). At baseline, 6998 (77.6%) patients were reported to have ASCVD. The Portuguese cohort had lower proportions of investigator-reported very high CV risk individuals (56.4%) and patients with ASCVD (60.7%). Moreover, mean (SD) LDL-C levels at baseline were lower among patients enrolled in Portugal: 80.8 (32.8) mg/dL vs. 93.0 (46.7) mg/dL in all other countries (baseline analysis dataset; see Supplementary Table 3 for mean (SD) LDL-C levels at baseline in the LDL-C dataset). This observation was consistent irrespective of the category of investigator-reported CV risk: mean (SD) baseline LDL-C levels were 88.6 (37.1) mg/dL in the Portuguese cohort of high CV risk individuals compared with 103.8 (50.4) mg/dL in all other countries, and 74.7 (27.9) mg/dL in the Portuguese cohort of very high CV risk individuals compared with 88.7 (44.3) mg/dL in all other countries.
Compared with the group of all other countries, the proportion of patients not on any LLT at baseline was lower in Portugal (6.0% vs. 21.1%), and the proportion of patients taking combination LLT at baseline was higher in Portugal (43.6% vs. 25.3%) (Table 2 and Supplementary Table 4). At one-year follow-up, the proportion of patients on a single LLT was lower in Portugal (45.3%) compared with the group of all other countries (57.2%); conversely, the proportion of patients on combination LLT was higher in Portugal (50.4%) compared with the group of all other countries (37.8%); the proportion of patients not on any LLT was similar in Portugal (2.6%) and in the group of all other countries (3.3%). None of the patients enrolled in Portugal were taking a PSCK9 inhibitor either at baseline or at one-year follow-up; in the group of all other countries, the proportion of patients taking a PCSK9 inhibitor either alone or in combination with at least one other LLT was 6.4% at baseline and increased to 8.9% at one-year follow-up. From baseline to one-year follow-up, LLT intensity less often was escalated and more often de-escalated in Portugal (12.8% escalated and 6.0% de-escalated) than in the group of all other countries (26.3% escalated and 2.2% de-escalated) (Table 4 and Supplementary Table 5). Moreover, LLT intensity more often remained unchanged in Portugal (79.5%) compared with the group of all other countries (70.3%).
In the LDL-C dataset, mean (SD) LDL-C levels at one-year follow-up were slightly higher in the Portuguese cohort: 81.6 (36.1) mg/dL vs. 76.7 (36.6) mg/dL in all other countries (Table 6 and Supplementary Table 3). This observation was consistent irrespective of the category of investigator-reported CV risk: mean (SD) baseline LDL-C levels were 90.1 (36.9) mg/dL in the Portuguese cohort of high CV risk individuals compared with 88.6 (39.8) mg/dL in all other countries, and 75.2 (34.4) mg/dL in the Portuguese cohort of very high CV risk individuals compared with 72.0 (34.1) mg/dL in all other countries. In the LDL-C dataset, between baseline and one-year follow-up, the mean LDL-C level increased 0.6 mg/dL (from 81.0 mg/dL to 81.6 mg/dL) in the Portuguese cohort; on the contrary, it declined 17.0 mg/dL (from 93.7 mg/dL to 76.7) in the group of all other countries. The corresponding changes in mean LDL-C levels between baseline and one-year follow-up were a decline of 0.1 mg/dL vs. a decline of 17.5 mg/dL, respectively, in high CV risk patients, and an increase of 1.1 mg/dL vs. a decline of 16.8 mg/dL, respectively, in very high CV risk patients.
In the Portuguese cohort, the proportion of patients at LDL-C goal declined from 30.4% at baseline to 22.6% at one-year follow-up; similarly, LDL-C goal attainment declined from 34.1% to 22.7% in high CV risk patients and from 27.6% to 22.4% in very high CV risk patients (Table 6). In contrast, in the group of all other countries, the proportion of patients at LDL-C goal increased from 21.1% at baseline to 31.0% at one-year follow-up; LDL-C goal attainment increased from 24.1% to 31.2% in high CV risk patients and from 19.9% to 31.0% in very high CV risk patients (Supplementary Table 3).
DiscussionIn the Portuguese cohort of the largest European observational study investigating LLT use and LDL-C goal attainment, conducted after the publication of the 2019 ESC/EAS guidelines for the management of dyslipidemia, we observed minor changes in mean LDL-C levels in both high- and very high-risk patients over one year of longitudinal follow-up, despite a reduction in the number of patients not taking any LLT and an increase in the proportion of patients taking a combination of a statin and ezetimibe. Over the same period, LDL-C goal attainment declined in both high-risk and very high-risk patients, to a degree that exceeded what would be anticipated based on the change in mean LDL-C levels alone.
These findings differ markedly from those observed in the patient cohort from the other 13 countries participating in the SANTORINI study, where mean LDL-C levels decreased by 17 mg/dL and the proportion of patients achieving LDL-C goals increased 1.47-fold over one year. Although a greater proportion of patients in Portugal were at goal at baseline compared to the other countries (30.4% vs. 21.1%), this trend reversed at one-year follow-up, with more patients outside Portugal achieving LDL-C targets.
Several factors may have contributed to the observed discrepancies between the Portuguese cohort and the patient populations from other participating countries. First, the mean baseline LDL-C level was lower in the Portuguese cohort (81.0 mg/dL) compared to the group of patients from the other participating countries (93.7 mg/dL). This difference may, in part, be explained by a lower proportion of patients not receiving any LLT and a higher proportion receiving combination LLT in the Portuguese cohort at baseline. Second, the Portuguese cohort included fewer patients with investigator-reported ASCVD (60.7% vs. 77.6%) and fewer classified as being at very high CV risk (56.4% vs. 71.4%) compared to patients from the other participating countries. Consequently, due to this more favorable risk profile and the lower mean baseline LDL-C level, patients in the Portuguese cohort were, a priori, more likely to be closer to or already at the recommended LDL-C target at baseline. In fact, a higher proportion of Portuguese patients had already achieved the LDL-C goal at baseline compared with all other patients. For the same reasons, the need for LLT intensification was lower in Portugal, which may partly explain the lower rate of LLT escalation observed between baseline and one-year follow-up in the Portuguese cohort compared to that of the other countries (12.8% vs. 26.3%). Third, although combination LLT was more frequently used at one-year follow-up in Portugal compared to other countries (50.4% vs. 37.8%), a notable proportion of patients in the latter group (6.7%) received a PCSK9 inhibitor in combination with at least one other LLT, whereas no patients in the Portuguese cohort were treated with a PCSK9 inhibitor. The potent LDL-C–lowering effect of PCSK9 inhibitors, especially when used in combination regimens, may have contributed to the higher proportion of LDL-C goal attainment at one-year follow-up in patients from other countries compared with those from Portugal (31.0% vs. 22.6%). The absence of PCSK9 inhibitor use in the Portuguese cohort may reflect the lower proportion of patients with investigator-reported ASCVD at baseline, as current guidelines provide weaker recommendations for PCSK9 inhibitor use in primary prevention settings compared with secondary prevention.12 Fourth, although LLT de-escalation was infrequent overall, it occurred more often in Portugal compared to other countries (6.0% vs. 2.2%). This difference may have further contributed to the lower proportion of patients achieving LDL-C goals at one-year follow-up in the Portuguese cohort. Moreover, despite the lower mean LDL-C level at baseline in the Portuguese group, the combination of more frequent LLT de-escalation and less frequent therapy escalation may help explain the higher mean LDL-C level observed at follow-up in this cohort compared to patients from the other participating countries.
Another notable observation in the Portuguese cohort is the disproportionately large reduction in the proportion of patients at LDL-C goal from baseline to one-year follow-up (30.4% to 22.6%), despite only a minimal increase in mean LDL-C levels during this period (81.0–81.6 mg/dL). This finding suggests that many patients who were at LDL-C goal at baseline had LDL-C levels close to the upper limit of the guideline-recommended targets (55 mg/dL for very high-risk and 70 mg/dL for high-risk individuals). As such, even a slight increase in LDL-C levels may have been sufficient to shift a substantial proportion of these patients out of goal range.
The comparison between the Portuguese cohort and patients from other countries underscores two key practical points. First, patients in the Portuguese cohort were more frequently on LLT at baseline, particularly combination therapy, which corresponded with a higher rate of LDL-C goal achievement. This observation is consistent with prior research19 and the overall findings of the SANTORINI study,18 supporting the superior efficacy of combination LLT over statin monotherapy in reaching LDL-C targets. Consequently, many experts recommend shifting clinical practice toward initiating high-intensity combination LLT rather than relying solely on high-intensity statin monotherapy to better mitigate residual CV risk associated with inadequate LDL-C management.20 Second, during the one-year follow-up period, LLT was escalated in only 12.8% of patients in Portugal, compared with 26.3% in the other countries. This indicates a higher prevalence of therapeutic inertia in the Portuguese cohort, with a ratio of LLT intensification to non-intensification of 1:6.7, vs. 1:2.8 in the other group. Strategies proven to reduce therapeutic inertia and improve LDL-C control include multidisciplinary, team-based collaborative care.21 These approaches can help address common barriers, such as limited physician time, by involving other healthcare professionals – including nurses and pharmacists – in patient education regarding medication intensification, adherence, and side effect management. However, current implementation research has limitations, including predominantly single-center studies, methodological weaknesses, and insufficient evaluation of innovative tools like artificial intelligence. Therefore, further high-quality implementation research is needed to identify effective, sustainable interventions to optimize lipid management in the long term.
The rate of LDL-C goal attainment in the Portuguese cohort of the SANTORINI study compares favorably with prior studies conducted in Portugal, including DYSIS-Portugal,22 DISGEN,23 Portuguese cohort of patients with CHD in EUROASPIRE V,24 LATINO,25 PORTRAIT-DYS,26 and LATINO-ACS.27 Nevertheless, mean LDL-C levels at one-year follow-up remained substantially above guideline-recommended thresholds: 90.1 mg/dL in high-risk patients and 75.2 mg/dL in very high-risk patients – approximately 20 mg/dL above their respective targets. Based on the well-established relationship between LDL-C levels and CV outcomes, this 20 mg/dL excess corresponds to an estimated 10% increase in relative risk of major vascular events over five years, representing a missed opportunity to prevent avoidable and potentially life-threatening events. A 22% reduction in mean LDL-C would be needed to achieve the 70 mg/dL target in high-risk patients, and a 27% reduction would be required to reach the 55 mg/dL goal in very high-risk patients. Clinical studies have demonstrated that adding bempedoic acid to maximally tolerated statin therapy can reduce LDL-C levels by 17–23% in patients at high or very high CV risk.28 Notably, no patients in the Portuguese cohort received bempedoic acid during the study period. Incorporating this therapy into treatment strategies may help a substantial proportion of patients not at goal to achieve guideline-recommended LDL-C levels.
Several limitations of this study must be acknowledged. First, although recruitment was conducted across multiple sites by physicians from various specialties and broad inclusion criteria were applied, the relatively small sample size may limit both the internal and external validity of the findings. To address this, 95% confidence intervals have been provided for the proportions of patients achieving LDL-C targets. Second, as sites involved in clinical research often differ systematically from those that do not participate, the results may reflect a best-case scenario and may not be fully generalizable to routine clinical practice. Third, CV risk may have been underestimated in some patients classified as very high risk. It remains unclear whether this potential misclassification led to physicians targeting an LDL-C goal of 70 mg/dL rather than the recommended 55 mg/dL, which could have artificially inflated the observed rate of LDL-C goal attainment. Fourth, the definition of LDL-C goal attainment was based solely on absolute risk-based thresholds (70 mg/dL and 55 mg/dL), without considering the additional guideline-recommended criterion of at least a 50% reduction from baseline levels. Finally, given the observational, non-interventional, and cross-sectional design of the study, medication adherence was neither systematically assessed nor supported through targeted interventions, limiting insight into real-world treatment effectiveness.
ConclusionIn summary, the Portuguese cohort of the SANTORINI study reveals both meaningful progress and ongoing challenges in real-world dyslipidemia management following the 2019 ESC/EAS guidelines. At baseline, the greater use of combination LLT with statins and ezetimibe, coupled with reduced reliance on statin monotherapy, was associated with superior LDL-C goal attainment compared to other European cohorts. However, over the one-year follow-up period, LDL-C goal achievement declined among both high- and very high-risk patients, diverging from the improvements observed in other participating countries. This unfavorable trend likely reflects suboptimal treatment intensification, limited integration of adjunctive therapies such as PCSK9 inhibitors and bempedoic acid, and persistent clinical inertia. These findings underscore the urgent need to optimize guideline-concordant lipid management in Portugal by expanding access to potent combination LLT and implementing comprehensive strategies to address therapeutic inertia. Enhancing LDL-C control in high- and very high-risk patients represents a pivotal opportunity to reduce preventable CV events and improve long-term clinical outcomes.
FundingSANTORINI was funded by Daiichi Sankyo Europe GmbH, Munich, Germany. The funder had a role in the study design, data collection, data analysis, and interpretation. Moreover, the funder provided medical writing support in accordance with Good Publication Practice.
Conflicts of interestCarlos Aguiar has received payments for speaker services, consultancy, and research activities from the following entities: Abbott, Abbvie, Amgen, Alnylam, AstraZeneca, Bayer, BiAL, Boehringer Ingelheim, Daiichi Sankyo, Ferrer, Gilead, GSK, Lilly, Novartis, Novo Nordisk, Pfizer, Sanofi, Servier, Takeda, Tecnimede. Patrício Aguiar received grants/research support from Takeda, and honoraria from Takeda, Alexion, Alnylam, Sanofi, Amicus, Biomarin, Ultragenyx, Chiesi, MSD, Bayer, Daiichi Sankyo, Viartris, Menarini, Novartis, Amgen, and GSK. P.M. has received payments for speaker services, consultancy, and research activities from Daiichi Sankyo. Fausto Pinto reports receiving personal fees from Boehringer Ingelheim, Daichi Sankyo, Novartis, Servier, Vifor, and Zydus and serving on advisory boards for Daiichi Sankyo, Medtronic, Novartis, Servier, and CSL Vifor. Pedro von Hafe has received speaker fees from Boehringer Ingelheim, Astra Zeneca, Daichi Sankyo, and Novartis. Carla Teixeira and Jorge Ruivo are employees of Daiichi Sankyo. Alberico Catapano, in the last three years, has received honoraria, lecture fees or research grants from Aegerion, Akcea Therapeutics, Amarin, Amgen, Amryt Pharma, AstraZeneca, Daiichi Sankyo, Esperion, Ionis Pharmaceutical, Medscape Education, Menarini, Merck, Mylan, Novartis, Novo Nordisk, PeerVoice, Pfizer, Recordati, Regeneron, Sanofi, The Corpus, and Viatris. Kausik Ray reports unrestricted research grants (last three years) from Imperial College London, Amarin, Amgen, Sanofi, Regeneron, Daiichi Sankyo, and Ultragenix; Consulting fees from Novartis, Daiichi Sankyo, Kowa, Esperion, Novo Nordisk, MSD, Lilly, Silence Therapeutics, AZ, New Amsterdam Pharma, Bayer, Beren Therapeutics, CLEERLY, EMENDOBIO, SCRIBE, CRISPR, VAXXINITY, Amarin, Regeneron, Ultragenix, for serving as a member of the SC or EC of clinical trials and roles as PI, NLI, attending advisory boards; lecture fees from Novartis, BI, AZ, Novo Nordisk, Viatris, Amarin, Sanofi, Amgen, Esperion, Daiichi Sankyo, Macleod Pharma for CME and non-CME symposia at international meetings; stock options from New Amsterdam Pharma, SCRIBE and PEMI31. João Sequeira Duarte, Victor Gil, Jorge Mimoso, Fernando Pinto, and João Raposo report no conflicts of interest.