Secondary myocardial involvement by diffuse large B-cell lymphoma is a rare occurrence. Left ventricular (LV) twist is considered an essential part of LV function. In normal circumstances LV twist results from the movement of two orthogonally oriented muscular bands of a helical myocardial structure with consequent clockwise rotation of the base and counterclockwise rotation of the apex. Three-dimensional (3D) speckle-tracking echocardiography (3DSTE) has been found to be feasible for non-invasive 3D quantification of LV wall motion and rotational mechanics. The present report aimed to assess LV twisting motion in a patient with diffuse large B-cell lymphoma with positron emission tomography/computer tomography-proven cardiac involvement by 3DSTE. During 3DSTE, reduction in some segmental radial, longitudinal, circumferential, area and 3D LV strains were found. Apical and basal LV rotations were found to be in the same counterclockwise direction, confirming near absence of LV twist – so-called rigid body rotation.
O envolvimento do miocárdio secundário ao linfoma difuso de células grandes B é uma ocorrência rara. A torção ventricular esquerda (VE) é considerada uma parte essencial da função VE. Em circunstâncias normais, a torção do VE resulta do movimento de uma estrutura miocárdica helicoidal de duas bandas musculares orientadas ortogonalmente, com a consequente rotação no sentido dos ponteiros do relógio da base do VE e rotação contra os ponteiros do relógio do ápex do VE. A ecocardiografia tridimensional (3D) de speckle-tracking (3DSTE) foi considerada adequada para a quantificação 3D não invasiva da contratilidade segmentar VE e da mecânica de rotação. Este artigo tem como objetivo a avaliação do movimento de torção VE por 3DSTE num doente com linfoma difuso de células grandes B e envolvimento cardíaco comprovado por tomografia de emissão de positrões/tomografia. Por 3DSTE foi verificada uma certa redução do strain radial, longitudinal, circunferencial do VE. A rotação dos segmentos basais e apicais do VE foi semelhante, num mesmo sentido, contra os ponteiros do relógio. Tal conduziu a uma redução marcada da torção do VE – rigid body rotations. Detetaram-se rotações VE apicais e basais no sentido contrário ao dos ponteiros do relógio, confirmando a ausência próxima da torção VE conforme referido «rotação rígida do corpo».
Secondary myocardial involvement by diffuse large B-cell lymphoma is an uncommon occurrence. Primary mediastinal B-cell lymphoma is an uncommon aggressive subset of diffuse large B-cell lymphoma.1 It frequently spreads locally from the thymus into the pleura and the pericardium, but rarely invades directly through the heart.1 It occasionally manifests as an intracardiac mass, often diagnosed on autopsy, and 90% are clinically silent.2,3 This type of lymphoma is more prevalent in women, mainly in the third and fourth decades of life, and represents 2-3% of non-Hodgkin's lymphomas.1,4 It usually presents with systemic symptoms (weight loss, fever, night sweats), shortness of breath, chest discomfort, and palpable lymph nodes.
Left ventricular (LV) twist is considered an essential part of LV function.5 In normal circumstances LV twist results from the movement of two orthogonally oriented muscular bands of a helical myocardial structure with consequent clockwise rotation of the LV base and counterclockwise rotation of the LV apex.6 This twisting deformation plays a fundamental part in the mechanical efficiency of the heart, resulting in 60% ejection fraction (EF) with only 15% fiber shortening.7 Recent developments in speckle-tracking echocardiography (STE) provide an opportunity to make accurate, reproducible and bedside assessments of LV twist in daily clinical practice.5 Three-dimensional (3D) speckle-tracking echocardiography (3DSTE) has been found to be feasible for non-invasive 3D quantification of LV wall motion and rotational mechanics.8 The present report aimed to assess LV twisting motion in a patient with diffuse large B-cell lymphoma with positron emission tomography (PET)/computer tomography (CT)-proven cardiac involvement by 3DSTE.
Case reportA 52-year-old female patient was admitted to the hospital due to shortness of breath. As a part of her examination, chest and abdominal CT was performed, on which several pathological lymph nodes could be observed in the mediastinal and abdominal regions together with pleural and pericardial effusions. In the upper third of the mediastinum, a soft tissue mass was detected, from which a biopsy was taken. Histology confirmed a CD20-positive primary mediastinal lymphoma, which is considered a subset of diffuse large B-cell lymphoma and is an uncommon clinical and pathological entity in the World Health Organization classification of lymphomas.1,4
Chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine and methylprednisolone (R-CHOP) was started according to international recommendations.9 R-CHOP includes rituximab, an anti-CD20 antibody, an essential part of the treatment. Following four cycles every 21 days, chest CT was repeated, which detected small lymph nodes in the hilar regions and in the right paratracheal area. Thus, according to the CT scan, the treatment resulted in partial response.
Altogether, the patient received eight cycles of such chemotherapy. After completion of the treatment, a combined low-dose plain CT and 18F-fluorodeoxyglucose (FDG)-PET examination was performed using a Siemens Biograph 6 PET/CT scanner (Siemens Healthcare, Erlangen, Germany). PET/CT showed activity in the mediastinal region and a single 2 cm×5 cm active lymph node in the left hilar region. Regarding disease activity, the patient received 39.6 Gy of radiation to the mediastinal area. Three weeks later, palpable masses could be detected during physical examination in the left and right lower limb region. Biopsy was performed of the left-side mass, showing extranodal manifestation of the disease. The patient went through two cycles of salvage chemotherapy (rituximab, cisplatin, cytosine arabinoside and dexamethasone, R-DHAP) and stem cell collection was also performed. Following treatment, the skin manifestations disappeared.
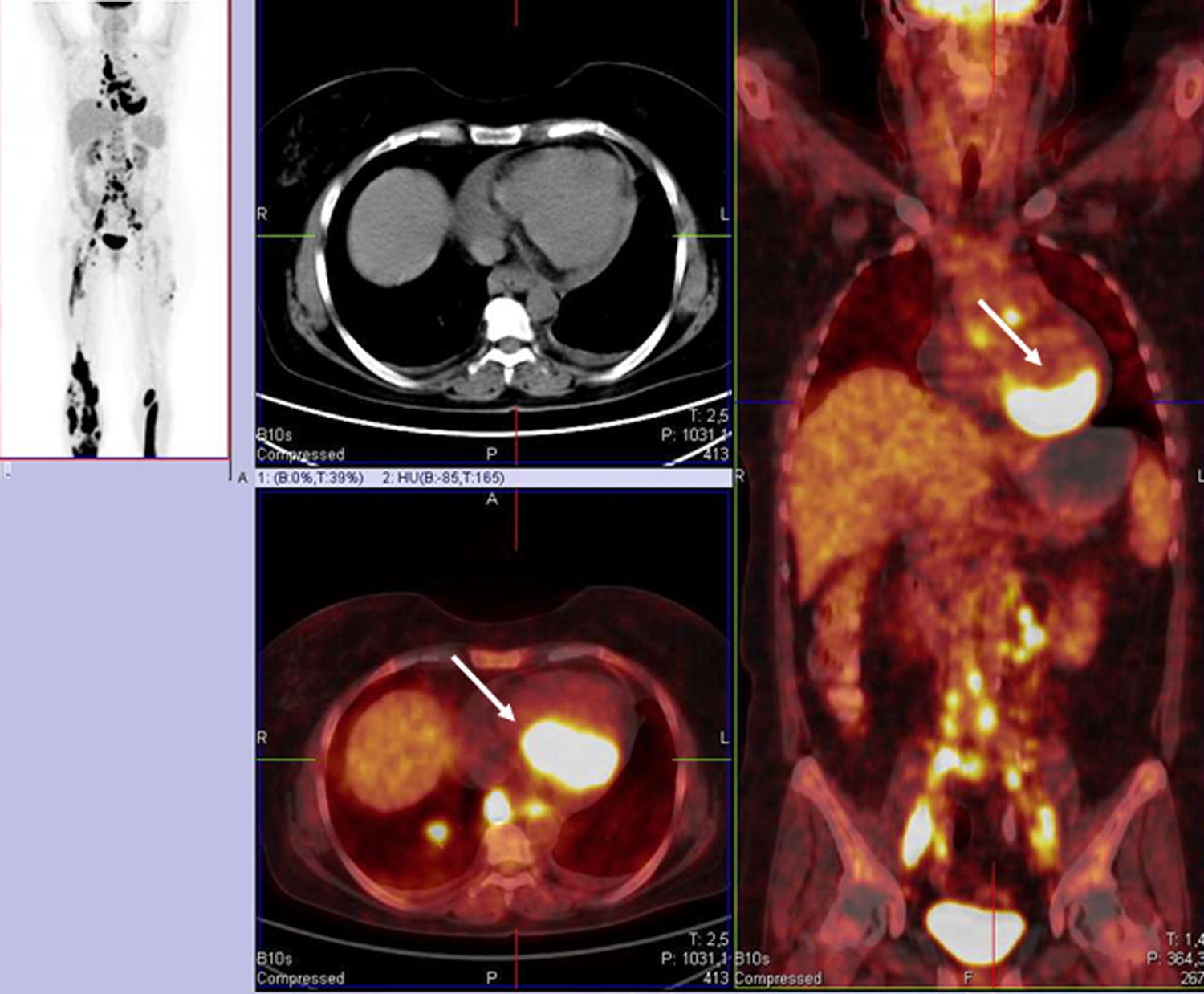
Autologous stem cell transplantation was planned as the next treatment option in the event of complete remission. To confirm the response, combined low-dose plain CT and 18F FDG-PET examination was repeated, which showed progression in the skin and the lymph node regions. Moreover, pericardial effusion (maximum thickness 22 mm) and cardiac involvement in the dorsal region (multiple active fields with the maximum standardized uptake value of 33.4) were also confirmed, indicating lymphoma manifestation in the heart (Figure 1).
No arrhythmias were detected, but the patient had symptoms of hypotonia and chest pain, as well as anemia (hemoglobin: 94 g/l) and increased lactate dehydrogenase (1824 U/l) and troponin T (0.261 μg/l) levels, with normal creatinine kinase (62 U/l). Urgent coronary angiography showed normal epicardial coronary arteries. Neurological symptoms including dizziness, blurred vision and altered mental status indicated central nervous system manifestation of the lymphoma. Plain non-enhanced cranial CT was performed, with a negative result. Lumbar puncture was also performed and showed no signs of lymphoma involvement or infection. A new course of chemotherapy was started with rituximab and the BFM protocol (first day: vincristine 2 mg, methotrexate 3 g/m2; from day 1-5: ifosfamide 800 mg/m2; day 4-5: cytosine arabinoside 2×150 mg/m2 and dexamethasone 10 mg/m2) for five days. Clinically, the patient is stable, but requires more courses of chemotherapy.
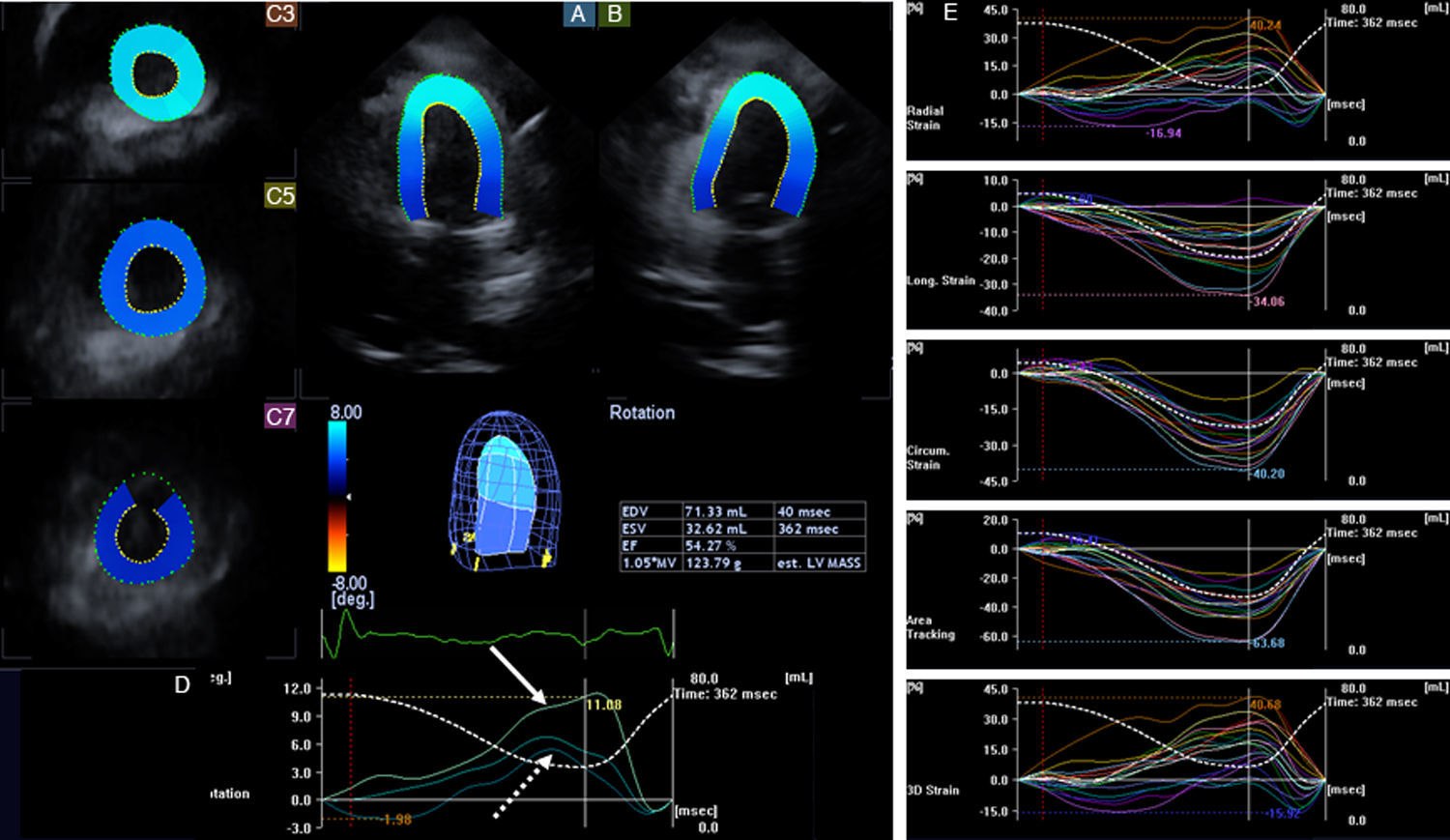
As a part of her assessment, routine two-dimensional Doppler echocardiography and 3DSTE were performed using Toshiba Artida™ ultrasound equipment with PST-30SBP phased-array and PST-25SX matrix phased-array transducers (Toshiba Medical Systems, Tokyo, Japan). Data acquisition and evaluation of echocardiographic studies followed recent guidelines and practices.8 Echocardiographic examination found normal atrial and ventricular dimensions and 50% EF, with no wall motion abnormalities and with grade I diastolic dysfunction. Color Doppler echocardiography confirmed grade 1 mitral regurgitation. Pericardial effusion was detected, 7-11 mm in size next to the posterior, lateral and inferior walls and 10-12 mm around the right apical region. During 3DSTE with a frame rate of 27 volumes per second, reduction in some segmental radial, longitudinal, circumferential, area and 3D LV strains were found (Figure 2). Apical and basal LV rotations were found to be in the same counterclockwise direction, confirming near absence of LV twist – so-called rigid body rotation (RBR).
Apical 4-chamber (A) and 2-chamber (B) views and short-axis views (C3, C5, C7) at different levels of the left ventricle (LV) extracted from the three-dimensional (3D) echocardiographic dataset in the patient with diffuse large B-cell lymphoma. The 3D model of the left ventricle and calculated LV volumetric and functional characteristics are also presented together with LV rotational (D) and strain (E) parameters. Apical (white arrow), midventricular and basal (dashed arrow) LV rotations were demonstrated to be in the same counterclockwise direction, confirming the near absence of LV twist (rigid body rotation). Some segmental LV strains were also found to be reduced. EDV: end-diastolic volume; EF: ejection fraction; ESV: end-systolic volume; LV: left ventricular.
The patient was enrolled in the MAGYAR-Path study (Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography in Pathological cases) and had provided informed consent. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institution's human research committee.
DiscussionPrimary cardiac tumors are extremely rare, with a prevalence of 0.001-0.28%.2,3 The incidence of secondary cardiac tumors varies from 2-18.3%.2,10 Among secondary tumors, cardiac involvement by lymphoma is infrequent, but in an autopsy study of malignant lymphoma, cardiac metastasis was found in 16% of cases.2,11 Less than 5% of cardiac tumors are primary, and of these, only 1.3% are primary cardiac lymphoma.12–15 Its incidence is higher in immunocompromised hosts, and B-cell lymphoma is the most common type.12
Despite involvement of myocardial tissue, cardiac symptoms may be absent or non-specific. Case reports seem to indicate that the heart is more often involved in non-Hodgkin's lymphoma, but pericardial manifestation is more common in Hodgkin's disease.2,13 Cardiac lymphomas are usually small and multiple, sometimes focal or diffuse, but tumor infiltrations of the pericardium, myocardium or endocardium have also been observed. The right side of the heart has been found to be more frequently involved than the left side.2
To the best of the authors’ knowledge this is the first time alterations in LV rotational mechanics have been demonstrated in a patient with PET/CT-proven cardiac lymphoma. The left ventricle consists of a 3D helical structure which is responsible for cyclic twisting deformation in systole, resulting from clockwise rotation of the LV base and counterclockwise rotation of the LV apex.6 It is known that aging appeared to be related to an increase in LV twist,16 and in specific pathological circumstances LV rotation and twist can be altered. In particular, in recent studies LV basal rotation was found to be moderately increased in hypertrophic cardiomyopathy, but was reduced in non-ischemic dilated cardiomyopathy due to reduction in both apical and basal LV rotations.5
In the present case, both apical and basal LV regions moved in the same counterclockwise direction to a similar extent (a difference of about 6°), confirming the near absence of LV twist (RBR). RBR has previously only been demonstrated in a limited number of disorders, including cardiomyopathies (peripartum,17 hypertensive,18 dilated with reduced EF19 and noncompaction20), infiltrative disorders (amyloidosis)21 and congenital heart disease (univentricular heart22 and hypoplastic right heart syndrome23). The underlying mechanism is unknown, but the findings presented could be explained by infiltration of the myocardium by lymphoma tissue, as confirmed in some areas by PET/CT. As the basic principle of PET is the increased glycolysis characteristic of tumors, which consume more glucose than normal tissue, the damage caused by chemotherapy shows up as a negative change on PET. Thus the PET/CT result and the 3DSTE result are identical, which proves that in this case the lesions are due to the lymphoma and not to cardiotoxic effects.
Further studies are needed to confirm our findings in larger patient populations focusing on the pathomechanism, clinical relevance and implications of LV RBR and on possible treatment options.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Financial support: This research received no specific grant from any funding agency, commercial or not-for-profit sectors.