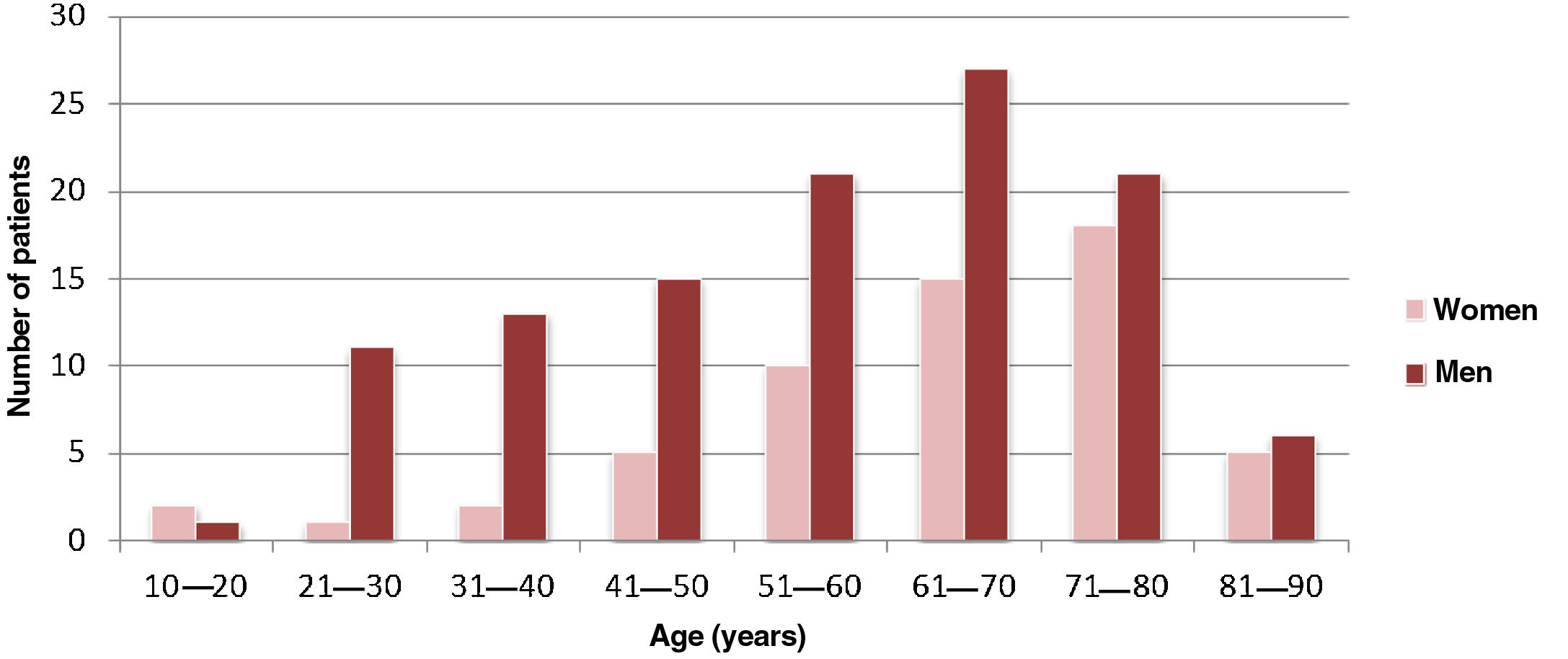Infective endocarditis (IE) is associated with high morbidity and mortality, despite advances in diagnosis and treatment.
ObjectiveTo assess changes in the epidemiological profile of IE, to perform a time-trend analysis and to define short-term and long-term prognostic predictors of IE.
MethodsRetrospective analysis of 173 patients admitted with a diagnosis of IE to a Portuguese level II Hospital between January 1998 and December 2013. The patients were divided into two groups according to the period of occurrence of the IE episode (1998-2007 vs. 2008-2013). The clinical event studied was the occurrence of death or the need for urgent surgery during hospitalization, and death in the follow-up period. Independent predictors of short-term and long-term prognosis were identified.
ResultsIn the first portion of the study, IE occurred in younger individuals, often drug addicts, users of intravenous drugs and with gastrointestinal disease, human immunodeficiency virus and hepatitis B infection. In the second portion of the study, IE occurred more frequently in individuals of an older age with concomitant cardiovascular disease; enterococcus was isolated more frequently. The independent predictors of in-hospital death or need for urgent valve surgery were septic shock and the occurrence of peri-annular complications. The independent predictors of long-term mortality were age, chronic kidney disease and IE due to multidrug-resistant microorganisms.
ConclusionDifferences were found in the epidemiological profile of IE during the study period. Referral for valve surgery increased slightly, but mortality remained high.
A endocardite infeciosa (EI) associa-se a elevada morbimortalidade, apesar dos avanços no diagnóstico e tratamento desta entidade.
ObjetivosAvaliar alterações no perfil epidemiológico da EI, realizar uma análise de tendência/evolução temporal e definir os preditores de prognóstico em curto e longo prazo.
MétodosAnálise retrospetiva de 173 doentes internados com EI num hospital português nível II, entre janeiro de 1998 e dezembro de 2013. Os doentes foram divididos em dois grupos, de acordo com o período de ocorrência da EI (1998-2007 versus 2008-2013). O evento clínico estudado foi a ocorrência de morte ou necessidade de cirurgia urgente durante o período de internamento e a morte no período de follow-up. Identificados os preditores independentes de prognóstico em curto e longo prazo.
ResultadosNo primeiro período do estudo a EI ocorreu em indivíduos mais jovens, frequentemente toxicodependentes, utilizadores de drogas intravenosas e com doença gastrointestinal, infeção por HIV e HBV. No segundo período do estudo esta patologia ocorreu mais em indivíduos de idade mais avançada, com patologia cardiovascular concomitante e o Enterococcus foi isolado mais frequentemente. Os preditores independentes de morte no internamento ou necessidade de cirurgia valvular urgente foram o choque séptico e a ocorrência de complicações perianulares. Os preditores independentes de mortalidade em longo prazo foram a idade, a doença renal crónica e a EI por microrganismos multirresistentes.
ConclusãoVerificaram-se diferenças no perfil epidemiológico da EI durante o período de estudo. A taxa de orientação para cirurgia valvular aumentou ligeiramente, mas a taxa de mortalidade manteve-se alta.
Infective endocarditis (IE) is an infection of the endocardium, caused by bacteria, fungi or other infectious agents.1 It can be classified according to its course (acute or subacute/chronic), the valve involved (native or prosthetic), the number of valves affected, the way it was acquired (community, intravenous drug users or healthcare-associated) and the microorganism involved.2
Despite advances in diagnosis and therapeutic approach, neither the incidence nor the mortality of IE has reduced in the last 30 years, and mortality remains high.3 IE incidence is 3–7/100 000 person-years, and in-hospital mortality ranges from 15–30%.2,4 In most epidemiological studies, the male-to-female ratio is ≥2:1; female patients have a worse prognosis and undergo valve replacement surgery less frequently.5
In recent years, there have been considerable changes in the epidemiology of IE, especially in industrialized countries. In the past, IE mainly affected young adults with known valve disease (mostly affected by rheumatic fever6–8); nowadays, the typical IE patient is elderly (peak incidence between 70–80 years) with valve disease of a degenerative etiology, prosthetic valve(s) or intracardiac devices, including pacemaker or cardioverter defibrillator.5,9 Other predisposing factors for IE have emerged, including intravenous drug use, invasive surgical procedures, vascular instruments, diabetes, long-term care (hemodialysis centers and outpatient parenteral antibiotic therapy programs).9,10
The microbiological profile of IE has also changed significantly. Classically, the agent most often involved in IE was Streptococcus viridans, causing subacute IE.11,12 More recent studies show that Staphylococcus aureus has been the most frequently isolated microorganism, manifesting itself clinically as acute IE.13
This major change in epidemiology, together with the variability in the forms of presentation of the disease, has had direct consequences on the diagnosis, treatment and prevention of IE. To overcome these difficulties, the modified Duke criteria are used, which are based on clinical, echocardiographic and microbiological findings, providing a sensitivity and specificity of approximately 80% for the diagnosis of IE.14
Recent advances in imaging techniques, including computed tomography, magnetic resonance imaging, and single-photon positron emission tomography have improved the identification of endocardial involvement and extracardiac complications of IE, resulting in the reformulation of the above diagnostic criteria.2 However, echocardiography and blood cultures remain cornerstones in the diagnosis of IE. Transthoracic (TTE) or transesophageal (TEE) echocardiography enable the identification of the main findings/criteria for IE (vegetation, abscess, and de novo dehiscence of a prosthetic valve).15 They are also useful for assessing disease severity, establishing prognosis, and assessing the effectiveness of antibiotic therapy.16 Positive blood cultures are reported in about 69% of all cases of IE, and IEs with negative blood cultures are principally related to the prior administration of antibiotics, sometimes delaying diagnosis and initiation of treatment.2
Rapid initiation of antibiotic therapy is essential to prevent major complications: heart failure (HF), stroke, systemic embolization, and sepsis/septic shock.17 In addition to prolonged antibiotic therapy regimens, the treatment of IE relies — in about half of the patients — on surgical removal of the infected tissue.18,19 Prognostically, IE is influenced by four main factors: patient characteristics, presence or absence of complications, infectious agent and echocardiographic findings.20 To date, four Portuguese studies have been published in which an attempt was made to retrospectively identify which factors are predictors of in-hospital mortality from IE.21–24 The development of sepsis, septic shock and HF were the common predictors of unfavorable prognosis in three of the studies.22–24 In-hospital mortality ranged from 13.1–31.3%. Only one of the studies assessed follow-up at six months24 and none of them studied long-term follow-up.
There are significant geographical variations and guidelines are often based on expert opinion due to the low incidence of IE, the lack of randomized trials and the limited number of meta-analyses.25 For this reason, and as it is a disease with such high mortality, the aims of this study were to assess the evolution of the epidemiological profile of IE before and after 2007 (regarding risk/predisposing factors, previous risk procedures, infectious agents), to assess the temporal trend of this disease, to define the current prognosis of affected patients, both in the short and long-term, and to define the main prognostic factors (clinical, analytical, echocardiographic and treatment).
MethodsType of studyObservational, retrospective study with a descriptive and an analytical component.
Study population and sampleThe study population included all patients admitted to a Portuguese level II hospital, which provides healthcare to approximately 1.2 million inhabitants and has an inpatient capacity of up to 704 beds, with a diagnosis of IE over 16 years (January 1998 to December 2013).
Inclusion criteria: Patients admitted to this hospital during the described period with a diagnosis of IE, using the modified Duke criteria.
Exclusion criteria: Inpatients with a diagnosis of IE who did not meet the modified Duke criteria.
The study variables are detailed extensively in appendixes 1, 2, and 3. They included demographic characteristics, the mode of IE acquisition, the presence of predisposing factors for IE (intracardiac prosthetic material, heart disease, or previous comorbidities), clinical presentation, analytical data, other diagnostic tests (echocardiogram and blood cultures), possible points of entry, IE prophylaxis, treatment, and complications.
The clinical events studied were the occurrence of in-hospital death or the need for urgent surgery and the occurrence of death at follow-up.
Data collectionInformation was obtained from the patient's paper medical files and electronic health records.
Ethical considerationsThe research protocol (SECVS 114/201) was submitted to and authorized by the Director of the Cardiology Department and the health ethics committee of the hospital in question. The entire research project complied with the rules of ethical conduct and good practice through compliance with the Declaration of Helsinki,26 the Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine,27 and was monitored according to good clinical practice guidelines.28
Statistical analysisThe data collected were recorded in a Microsoft Office Excel 2007® table and then uploaded to the IBM® Statistical Package for the Social Science (SPSS®) version 22.
Patients were divided into two groups according to the period in which they had IE, before and after 2007: G0 (n=93), patients who had IE between 1998-2007; G1 (n=80), patients who had IE between 2008-2013. The European IE guidelines published in 2009 marked a significant change with regard to the use of prophylaxis. Thus, the authors wanted to separate the groups around the year 2009. However, this separation caused a significant asymmetry, especially with regard to the number of patients in each group, so the year 2007 was chosen to make the groups more homogeneous and the break point remained close to the year 2009.
According to the central limit theorem, normal distribution was applicable for all statistical tests.29 Non-parametric tests were performed and revealed the same results as the parametric tests, so only the results of the parametric tests will be reported since they have greater statistical power.30
Descriptive statistics were used to analyze the baseline characteristics of the sample. Categorical variables were expressed as percentages and frequencies, and continuous variables as mean ± standard deviation. Differences between groups were compared using the Student's t test for independent samples for continuous variables and the chi-square (χ2) test or Fisher's exact test (‡, when the assumptions of the above were not met) for categorical variables. The association between the different variables with short- and long-term follow-up was initially tested with univariate analysis (Pearson's chi-square or Fisher's exact test for categorical variables and Student's t test for continuous variables). Univariate analysis of factors associated with inpatient death, inpatient or urgent surgery death, and death at follow-up was performed (Annexes 1, 2 and 3). It should be noted that patients who died during hospitalization were excluded from the analysis of the association between variables and death in long-term follow-up.
Using binary logistic regression, the independent predictors of death or urgent surgery in patients with IE were determined. To assess the prognosis and the independent predictors of death in the long-term, Cox regression was performed. The regression models included the variables considered clinically relevant and those that were significant in the previous univariate analysis, always taking into account the number of events in each univariate analysis to select only one variable per 10 events.31 Odds ratio (for the binary logistic regression model) and hazard ratio (for the Cox model) were calculated according to a 95% confidence interval (CI).
Survival of patients with IE was estimated using Kaplan-Meyer curves (95% CI) and differences between the two groups were assessed by a log-rank test. Statistical significance of p<0.05 and a 95% CI were considered.
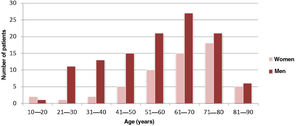
ResultsCharacterization of the sample and clinical characteristicsThe sample comprised 173 patients, 115 were male (66.5%), with a male-to-female ratio of approximately 2:1. The mean age of the patients was 58.7±17.4 years (Figure 1). In this study, 63.6% (n=110) of the IE cases were community-acquired, 27.8% (n=48) were healthcare-associated and 8.7% (n=15) related to intravenous drug abuse.
Group 0 (G0 - individuals with IE between 1998 and 2007) consisted of 93 patients (53.8% of IE patients) and group 1 (G1 - individuals with IE between 2008 and 2013) consisted of 80 patients (46.2% of IE patients).
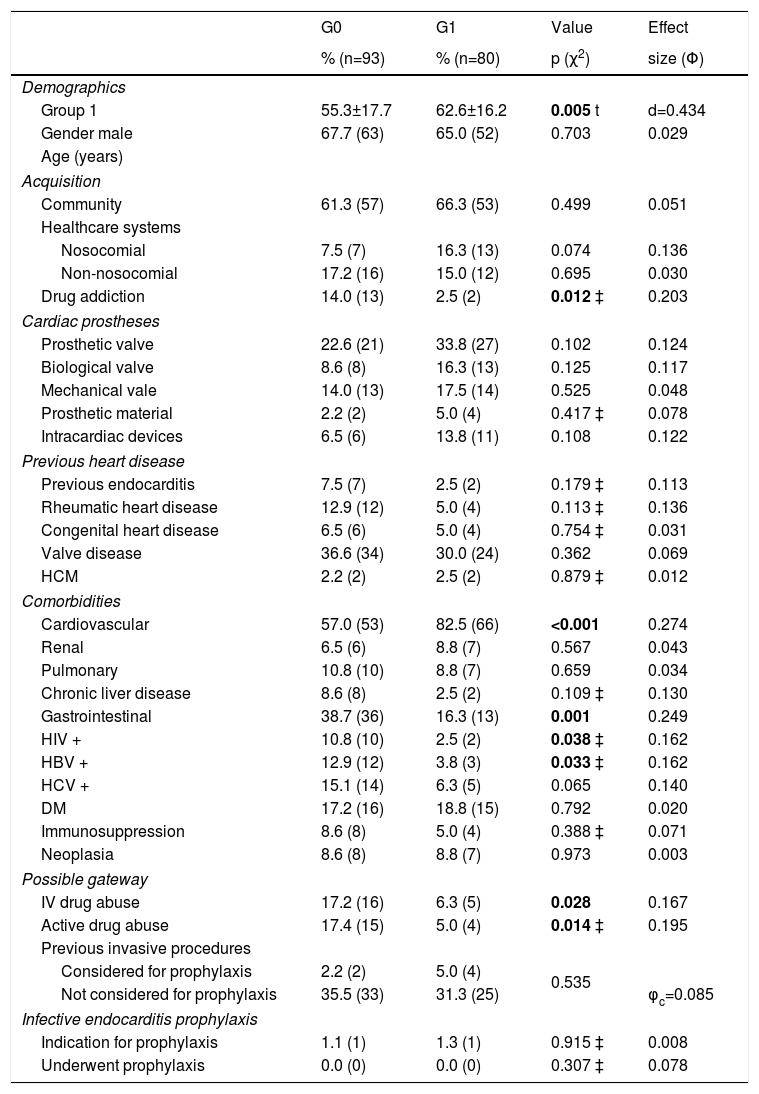
The comparison of baseline characteristics between groups 0 and 1 is summarized in Table 1. Regarding demographic characteristics, the gender distribution was similar in both groups. The G1 population, with a mean age of 62.6±16.2 years, was significantly older than the G0 population with a mean age of 55.3±17.7 years (t (171)=-2.83, p=0.005, d=0.43). As for the mode of acquisition, there were statistically significant differences between the two groups in the number of cases of IE associated with drug abuse (14.0% in G0 vs. 2.5% in G1 - χ2 (132)=2.86, p=0.012, Φ=0.203). Valvular disease was the most common cardiac predisposing factor (33.5%), but there were no significant differences between the two groups (χ2(1)=0.83, p=0.36, Φ=0.069).
Difference in baseline characteristics between G0 and G1 patients.
| G0 | G1 | Value | Effect | |
|---|---|---|---|---|
| % (n=93) | % (n=80) | p (χ2) | size (Φ) | |
| Demographics | ||||
| Group 1 | 55.3±17.7 | 62.6±16.2 | 0.005 t | d=0.434 |
| Gender male | 67.7 (63) | 65.0 (52) | 0.703 | 0.029 |
| Age (years) | ||||
| Acquisition | ||||
| Community | 61.3 (57) | 66.3 (53) | 0.499 | 0.051 |
| Healthcare systems | ||||
| Nosocomial | 7.5 (7) | 16.3 (13) | 0.074 | 0.136 |
| Non-nosocomial | 17.2 (16) | 15.0 (12) | 0.695 | 0.030 |
| Drug addiction | 14.0 (13) | 2.5 (2) | 0.012 ‡ | 0.203 |
| Cardiac prostheses | ||||
| Prosthetic valve | 22.6 (21) | 33.8 (27) | 0.102 | 0.124 |
| Biological valve | 8.6 (8) | 16.3 (13) | 0.125 | 0.117 |
| Mechanical vale | 14.0 (13) | 17.5 (14) | 0.525 | 0.048 |
| Prosthetic material | 2.2 (2) | 5.0 (4) | 0.417 ‡ | 0.078 |
| Intracardiac devices | 6.5 (6) | 13.8 (11) | 0.108 | 0.122 |
| Previous heart disease | ||||
| Previous endocarditis | 7.5 (7) | 2.5 (2) | 0.179 ‡ | 0.113 |
| Rheumatic heart disease | 12.9 (12) | 5.0 (4) | 0.113 ‡ | 0.136 |
| Congenital heart disease | 6.5 (6) | 5.0 (4) | 0.754 ‡ | 0.031 |
| Valve disease | 36.6 (34) | 30.0 (24) | 0.362 | 0.069 |
| HCM | 2.2 (2) | 2.5 (2) | 0.879 ‡ | 0.012 |
| Comorbidities | ||||
| Cardiovascular | 57.0 (53) | 82.5 (66) | <0.001 | 0.274 |
| Renal | 6.5 (6) | 8.8 (7) | 0.567 | 0.043 |
| Pulmonary | 10.8 (10) | 8.8 (7) | 0.659 | 0.034 |
| Chronic liver disease | 8.6 (8) | 2.5 (2) | 0.109 ‡ | 0.130 |
| Gastrointestinal | 38.7 (36) | 16.3 (13) | 0.001 | 0.249 |
| HIV + | 10.8 (10) | 2.5 (2) | 0.038 ‡ | 0.162 |
| HBV + | 12.9 (12) | 3.8 (3) | 0.033 ‡ | 0.162 |
| HCV + | 15.1 (14) | 6.3 (5) | 0.065 | 0.140 |
| DM | 17.2 (16) | 18.8 (15) | 0.792 | 0.020 |
| Immunosuppression | 8.6 (8) | 5.0 (4) | 0.388 ‡ | 0.071 |
| Neoplasia | 8.6 (8) | 8.8 (7) | 0.973 | 0.003 |
| Possible gateway | ||||
| IV drug abuse | 17.2 (16) | 6.3 (5) | 0.028 | 0.167 |
| Active drug abuse | 17.4 (15) | 5.0 (4) | 0.014 ‡ | 0.195 |
| Previous invasive procedures | ||||
| Considered for prophylaxis | 2.2 (2) | 5.0 (4) | 0.535 | |
| Not considered for prophylaxis | 35.5 (33) | 31.3 (25) | φc=0.085 | |
| Infective endocarditis prophylaxis | ||||
| Indication for prophylaxis | 1.1 (1) | 1.3 (1) | 0.915 ‡ | 0.008 |
| Underwent prophylaxis | 0.0 (0) | 0.0 (0) | 0.307 ‡ | 0.078 |
HCM: hypertrophic cardiomyopathy; HIV: human immunodeficiency virus; HBV: hepatitis B virus; HCV: hepatitis C virus; IV: intravenous; χ2: Chi-squared; ‡: Fisher's exact test; t: Student's t-test; Φ: Phi value; φc: Cramer's V; d: Cohen's d.
In bold, significant differences.
Of the comorbidities, gastrointestinal disease, human immunodeficiency virus and hepatitis B virus infections were more common in G0, while cardiovascular disease was more frequent in G1. In terms of possible points of entry, both a history of abuse and active IV drug use were more commonly detected in G0. The use of IE prophylaxis was similar in the two groups.
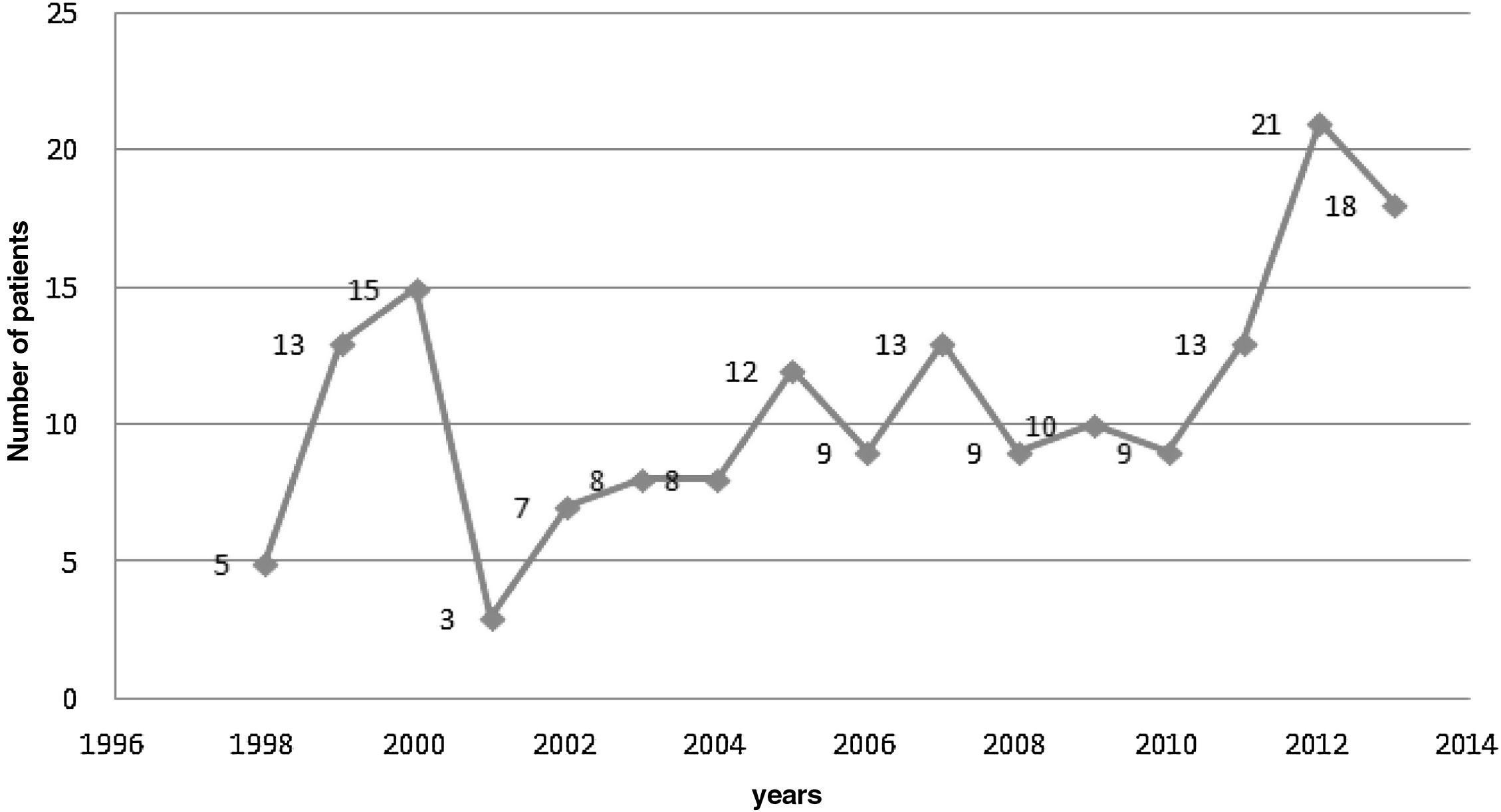
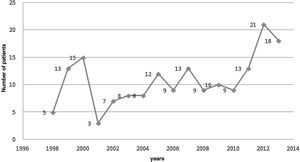
Evolution of the number of infectious endocarditis cases in the study periodFigure 2 reveals that the number of cases of IE admitted to the hospital where this study was carried out oscillated over the study period, reaching the lowest value in 2001, with only three affected patients, and the highest values in 2012 and 2013, with 21 and 18 patients affected, respectively, with an average of 10.8±4.65 cases/patients affected by IE per year.
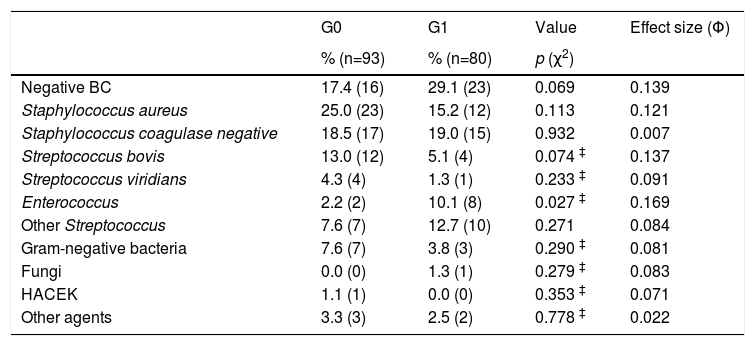
Microbiological profileA major proportion of blood cultures (22.5%) did not contain any infectious agent. Staphylococcus aureus was isolated in 35 patients (20.2%) and was the most frequently isolated microorganism, followed by Coagulase-negative Staphylococcus isolated in 32 patients (18.5%). However, there were no differences between the two groups regarding the isolation of these agents, whereas IE caused by Enterococcus occurred more frequently in G1 (2.2% in G0 vs. 10.1% in G1, χ2(1)=4.88, p=0.27, Φ=0.169) (Table 2).
Microbiological profile in 173 episodes of infective endocarditis.
| G0 | G1 | Value | Effect size (Φ) | |
|---|---|---|---|---|
| % (n=93) | % (n=80) | p (χ2) | ||
| Negative BC | 17.4 (16) | 29.1 (23) | 0.069 | 0.139 |
| Staphylococcus aureus | 25.0 (23) | 15.2 (12) | 0.113 | 0.121 |
| Staphylococcus coagulase negative | 18.5 (17) | 19.0 (15) | 0.932 | 0.007 |
| Streptococcus bovis | 13.0 (12) | 5.1 (4) | 0.074 ‡ | 0.137 |
| Streptococcus viridians | 4.3 (4) | 1.3 (1) | 0.233 ‡ | 0.091 |
| Enterococcus | 2.2 (2) | 10.1 (8) | 0.027 ‡ | 0.169 |
| Other Streptococcus | 7.6 (7) | 12.7 (10) | 0.271 | 0.084 |
| Gram-negative bacteria | 7.6 (7) | 3.8 (3) | 0.290 ‡ | 0.081 |
| Fungi | 0.0 (0) | 1.3 (1) | 0.279 ‡ | 0.083 |
| HACEK | 1.1 (1) | 0.0 (0) | 0.353 ‡ | 0.071 |
| Other agents | 3.3 (3) | 2.5 (2) | 0.778 ‡ | 0.022 |
BC: blood culture; HACEK: Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella; χ2: Chi-squared; ‡: Fisher's exact test; t: Student's t-test; Φ: Phi value.
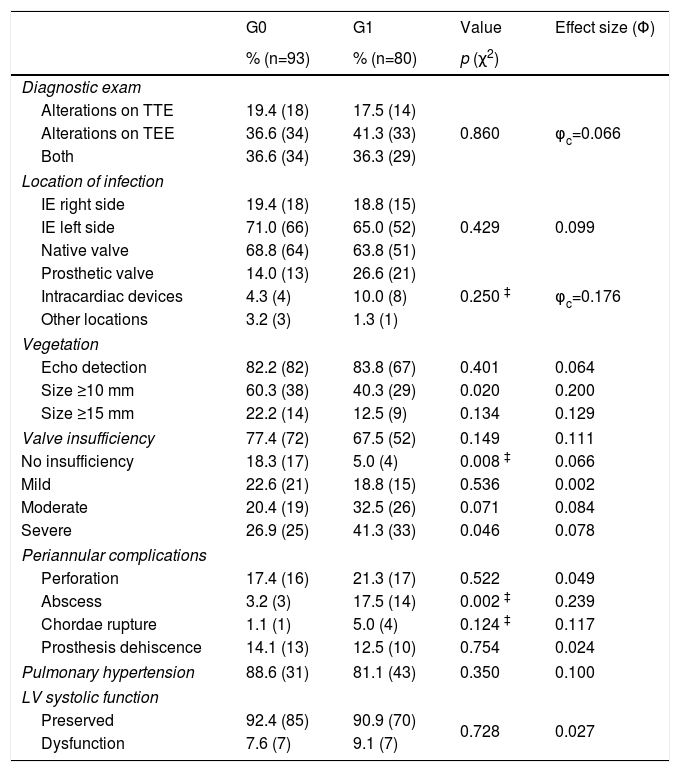
Echocardiography was used as an auxiliary diagnostic test in all patients. TEE was the test that contributed most to the diagnosis of IE, revealing changes suggestive of IE in 75.1% of patients, with no significant differences between the groups (Table 3).
Echocardiogram findings.
| G0 | G1 | Value | Effect size (Φ) | |
|---|---|---|---|---|
| % (n=93) | % (n=80) | p (χ2) | ||
| Diagnostic exam | ||||
| Alterations on TTE | 19.4 (18) | 17.5 (14) | ||
| Alterations on TEE | 36.6 (34) | 41.3 (33) | 0.860 | φc=0.066 |
| Both | 36.6 (34) | 36.3 (29) | ||
| Location of infection | ||||
| IE right side | 19.4 (18) | 18.8 (15) | ||
| IE left side | 71.0 (66) | 65.0 (52) | 0.429 | 0.099 |
| Native valve | 68.8 (64) | 63.8 (51) | ||
| Prosthetic valve | 14.0 (13) | 26.6 (21) | ||
| Intracardiac devices | 4.3 (4) | 10.0 (8) | 0.250 ‡ | φc=0.176 |
| Other locations | 3.2 (3) | 1.3 (1) | ||
| Vegetation | ||||
| Echo detection | 82.2 (82) | 83.8 (67) | 0.401 | 0.064 |
| Size ≥10 mm | 60.3 (38) | 40.3 (29) | 0.020 | 0.200 |
| Size ≥15 mm | 22.2 (14) | 12.5 (9) | 0.134 | 0.129 |
| Valve insufficiency | 77.4 (72) | 67.5 (52) | 0.149 | 0.111 |
| No insufficiency | 18.3 (17) | 5.0 (4) | 0.008 ‡ | 0.066 |
| Mild | 22.6 (21) | 18.8 (15) | 0.536 | 0.002 |
| Moderate | 20.4 (19) | 32.5 (26) | 0.071 | 0.084 |
| Severe | 26.9 (25) | 41.3 (33) | 0.046 | 0.078 |
| Periannular complications | ||||
| Perforation | 17.4 (16) | 21.3 (17) | 0.522 | 0.049 |
| Abscess | 3.2 (3) | 17.5 (14) | 0.002 ‡ | 0.239 |
| Chordae rupture | 1.1 (1) | 5.0 (4) | 0.124 ‡ | 0.117 |
| Prosthesis dehiscence | 14.1 (13) | 12.5 (10) | 0.754 | 0.024 |
| Pulmonary hypertension | 88.6 (31) | 81.1 (43) | 0.350 | 0.100 |
| LV systolic function | ||||
| Preserved | 92.4 (85) | 90.9 (70) | 0.728 | 0.027 |
| Dysfunction | 7.6 (7) | 9.1 (7) | ||
IE: infective endocarditis; TTE: transthoracic echocardiogram; TEE: transesophageal echocardiogram; PASP: pulmonary artery systolic pressure; LV: left ventricle; χ2: Chi-squared; ‡: Fisher's exact test; Φ: Phi value; φc: Cramer's V.
The presence of valvular insufficiency, specifically severe grade regurgitation, and the presence of abscesses were more commonly found in G1. No statistically significant differences were found between the two groups based on the contribution of the two types of echocardiogram (TTE and TEE) to the diagnosis of IE, the location of infection, the development of pulmonary hypertension or the existence of left ventricular systolic dysfunction.
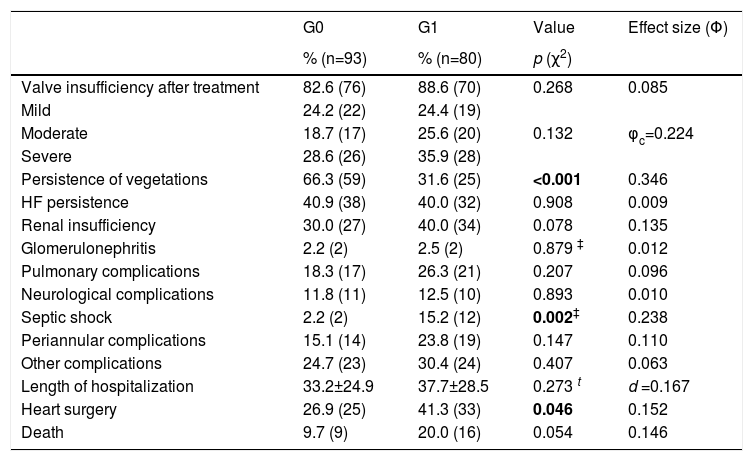
In-hospital evolutionOverall in-hospital mortality was 14.5%, with no significant differences between the two groups (Table 4).
Clinical events during in-hospital evolution in 173 patients.
| G0 | G1 | Value | Effect size (Φ) | |
|---|---|---|---|---|
| % (n=93) | % (n=80) | p (χ2) | ||
| Valve insufficiency after treatment | 82.6 (76) | 88.6 (70) | 0.268 | 0.085 |
| Mild | 24.2 (22) | 24.4 (19) | ||
| Moderate | 18.7 (17) | 25.6 (20) | 0.132 | φc=0.224 |
| Severe | 28.6 (26) | 35.9 (28) | ||
| Persistence of vegetations | 66.3 (59) | 31.6 (25) | <0.001 | 0.346 |
| HF persistence | 40.9 (38) | 40.0 (32) | 0.908 | 0.009 |
| Renal insufficiency | 30.0 (27) | 40.0 (34) | 0.078 | 0.135 |
| Glomerulonephritis | 2.2 (2) | 2.5 (2) | 0.879 ‡ | 0.012 |
| Pulmonary complications | 18.3 (17) | 26.3 (21) | 0.207 | 0.096 |
| Neurological complications | 11.8 (11) | 12.5 (10) | 0.893 | 0.010 |
| Septic shock | 2.2 (2) | 15.2 (12) | 0.002‡ | 0.238 |
| Periannular complications | 15.1 (14) | 23.8 (19) | 0.147 | 0.110 |
| Other complications | 24.7 (23) | 30.4 (24) | 0.407 | 0.063 |
| Length of hospitalization | 33.2±24.9 | 37.7±28.5 | 0.273 t | d =0.167 |
| Heart surgery | 26.9 (25) | 41.3 (33) | 0.046 | 0.152 |
| Death | 9.7 (9) | 20.0 (16) | 0.054 | 0.146 |
IE: infective endocarditis; HF: heart failure; χ2: Chi-squared; ‡: Fisher's exact test; Φ: Phi value; φc: Cramer's V; φc: Cramer's V; d: Cohen's d.
The most common complications during hospitalization were valvular insufficiency (84.4%), persistent vegetation (48.6%) and persistent HF (40.5%).
The development of septic shock and the need for referral to cardiac surgery was more common in G1 patients. No significant differences were found between the two groups for other clinical events during hospitalization.
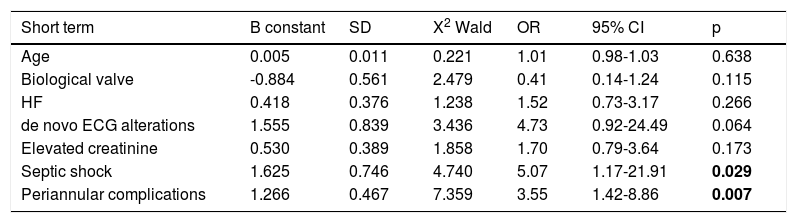
Assessment of short-term prognosisThe variables that were significant in the previous univariate analysis were included in the regression model (Appendix 2). By analyzing the independent predictors of short-term prognosis (Table 5), in-hospital mortality or urgent surgery was found to be significantly influenced by the development of complications such as septic shock and peri-annular complications. Septic shock was the complication that most frequently led to death or a need for urgent surgery, increasing the risk of these events by about five-fold (Bsepticshock=1.625, p=0.029).
Independent predictors of death in-hospital or urgent surgery (short-term follow-up) and death in long term follow-up.
| Short term | B constant | SD | X2 Wald | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| Age | 0.005 | 0.011 | 0.221 | 1.01 | 0.98-1.03 | 0.638 |
| Biological valve | -0.884 | 0.561 | 2.479 | 0.41 | 0.14-1.24 | 0.115 |
| HF | 0.418 | 0.376 | 1.238 | 1.52 | 0.73-3.17 | 0.266 |
| de novo ECG alterations | 1.555 | 0.839 | 3.436 | 4.73 | 0.92-24.49 | 0.064 |
| Elevated creatinine | 0.530 | 0.389 | 1.858 | 1.70 | 0.79-3.64 | 0.173 |
| Septic shock | 1.625 | 0.746 | 4.740 | 5.07 | 1.17-21.91 | 0.029 |
| Periannular complications | 1.266 | 0.467 | 7.359 | 3.55 | 1.42-8.86 | 0.007 |
| Long term | B constant | SD | X2 Wald | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| Age | 0.028 | 0.011 | 6.961 | 1.02 | 1.01-1.05 | 0.008 |
| Prosthetic valve | -0.523 | 0.326 | 2.577 | 0.59 | 0.31-1.12 | 0.108 |
| Chronic kidney disease | 1.174 | 0.429 | 7.492 | 3.23 | 1.40-7.49 | 0.006 |
| Multidrug-resistant agent | 0.654 | 0.307 | 4.531 | 1.92 | 1.05-3.51 | 0.033 |
| Gastrointestinal disease | 0.077 | 0.297 | 0.067 | 1.08 | 0.60-1.93 | 0.796 |
| HF | 0.495 | 0.290 | 2.918 | 1.64 | 0.93-2.89 | 0.088 |
| Left-sided vegetation | 0.119 | 0.425 | 0.078 | 1.13 | 0.49-2.59 | 0.780 |
SD: standard deviation; OR: odds ratio; HF: heart failure; HR: hazard ratio; CI: confidence interval; ECG: electrocardiogram.
The result of the univariate analysis concerning factors associated with long-term mortality is shown in Appendix 3. The independent predictors of long-term prognosis are shown in Table 5. Age, in contrast to short-term follow-up, proved to be an independent predictor of long-term death. In addition, the presence of chronic kidney disease and infection with multidrug-resistant microorganisms were significantly associated with long-term death. Chronic kidney disease was the independent predictor of long-term death with the highest relative risk (Bchronic kidney disease=1.174, p=0.006).
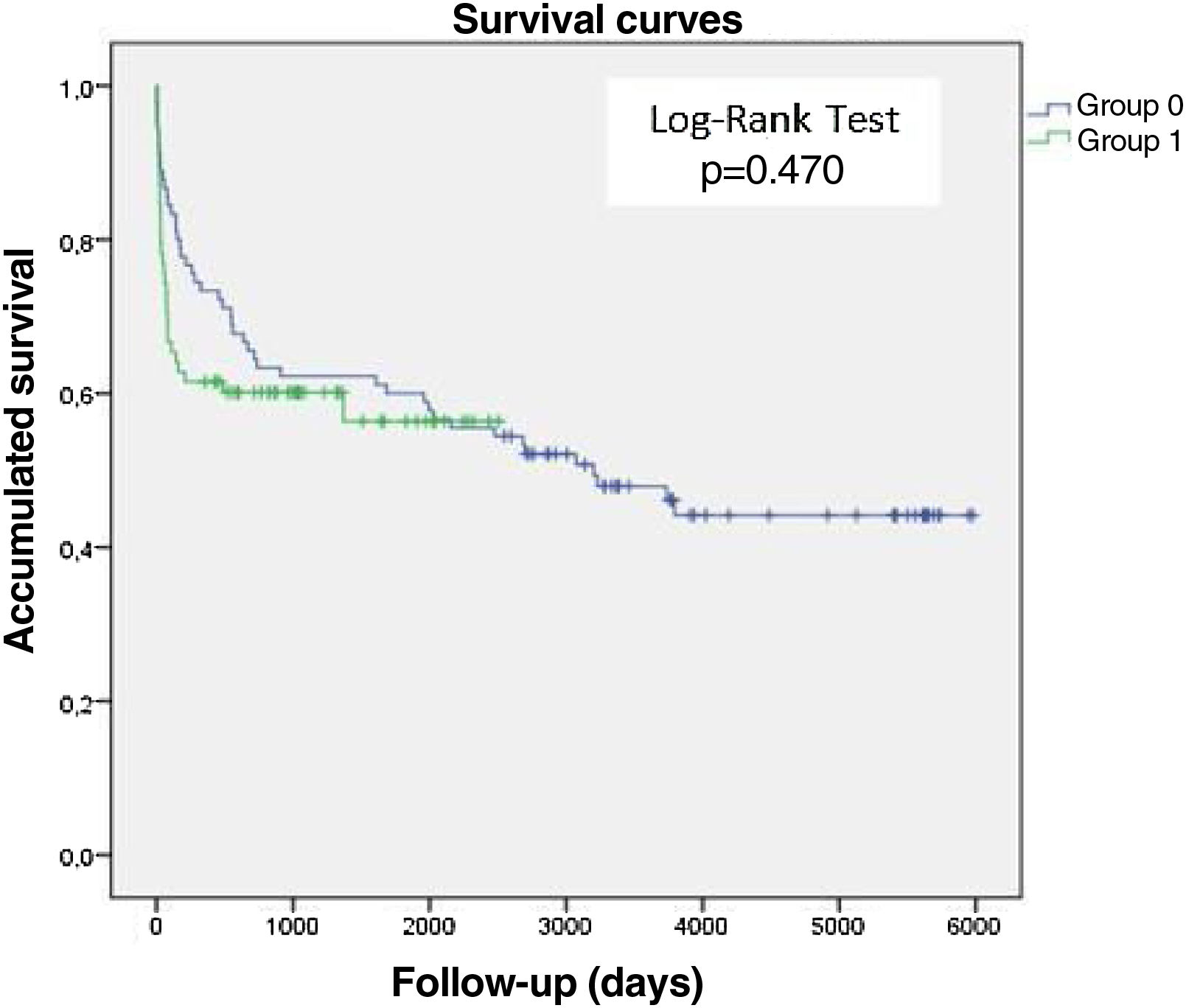
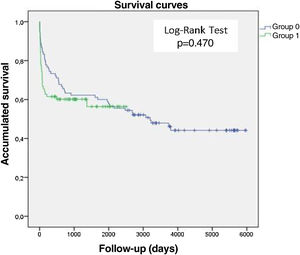
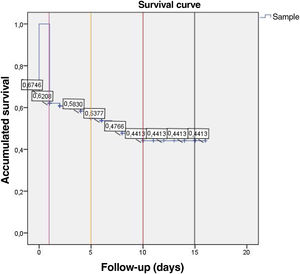
Survival analysis of patients with infective endocarditisIn this study, 97.7% of patients were followed up (Appendices 3 and 5) with a mean follow-up time of 1456 days. The Kaplan-Meyer survival curves, Figure 3, show that there was no significant difference between the two groups studied in terms of survival time after the IE episode (Log-Rank Test, p=0.470). G0 (blue line), the group from the first study period consequently had a longer follow-up period than G1 (green line), hence the difference in the length of the curves between the two groups (t(116)=7.41, p<0.001, d=1.12). The survival analysis curve for all patients with IE (Figure 4) reveals that mortality after one and five years was 38% and 47%, respectively. Mortality after 10 and 15 years appeared to be similar, at approximately 56%.
DiscussionIn this study, there was an annual oscillation in the number of patients hospitalized for IE, with a period with a higher number of cases between 2010/2011 and 2013. Males were the most affected individuals and valvular disease was the most common cardiac predisposing factor (33.5%).
In the first study period, IE occurred in younger individuals with active IV drug use, gastrointestinal disease, and concomitant HIV and HBV infection. In the second study period, IE occurred in older individuals with concomitant cardiovascular disease.
The most frequently isolated microorganism throughout the study period was Staphylococcus aureus. Enterococcus emerged significantly as the agent most responsible for IE in the second subperiod of the study.
Overall mortality associated with IE was 14.5% during hospitalization, 38% after one year, and 47% after five years. The referral rate for cardiac surgery was 34%, increasing in the second period of the study.
Independent predictors of in-hospital death or need for urgent surgery were septic shock and the occurrence of peri-annular complications. Independent predictors of long-term mortality were age, chronic kidney disease, and IE from multidrug-resistant microorganisms.
As this is not a population-based study, it was not possible to provide data regarding the incidence of IE over time. There was, however, an oscillation in the number of cases/year with a higher number of cases between 2010/2011 and 2013.
Data regarding on the incidence of IE have been controversial, and several studies have identified no change in the incidence of IE.4,32–35 However, some European population-based studies have shown an increase in the incidence of IE over time.36–38 This also occurred in a Spanish multicenter study, which included about 16 867 IE episodes over 12 years (2003-2014) and reported an increase in IE incidence of 2% yearly, from 2.72 in 2003 to 3.49 per 100 000 person-years in 2014, which was more evident in the older population.39
The increased incidence of IE in older populations was also reported by Hill et al.,40 who highlighted that half of all cases of IE in the United States of America and Europe occur in patients >60 years, and that the mean age of patients has increased steadily over the past 40 years. This increase seems to be represented in our study population as well, since the mean age of the population in the group that had endocarditis between 2008 and 2013 was higher than the group that had endocarditis between 1998-2007. The explanation for the increasing number of cases of IE in the older population may lie in the increased average life expectancy of the population and also in their greater contact with health services and with invasive techniques/procedures. In addition, more elderly populations also have a higher percentage of patients with valvular prostheses and intracardiac devices, such as pacemakers and cardioverter-defibrillators. This scenario was also represented in this study, where a higher percentage of patients with IE who had intracardiac devices/pacemakers or cardioverter-defibrillators was observed in the group that had endocarditis between 2008 and 2013. There was also a higher percentage of patients with prosthetic valves in this subgroup, although without statistical significance.
This study revealed a higher number of cases of IE in males (66.5%), with a male-to-female ratio of approximately 2:1, comparable to the results obtained by other studies.41–43
The microbiological profile showed that blood cultures in this study were positive in 76.3% of cases, a result consistent with other international studies.2,39 The most frequently isolated microorganism was Staphylococcus aureus (20.2%), as documented in most studies, and there was an increase in the number of Enterococcus-associated IE cases in the second study period. The increase in the number of Enterococcus-associated IE cases was also reported in the Spanish multicenter study39 and is likely related to the previously mentioned increase in the prevalence of invasive healthcare-related procedures in recent years, such as intravenous lines, catheterization, hemodialysis, etc.
Septic shock, a recognized unfavorable prognostic factor associated with high mortality in IE,44–46 occurred more frequently in the subgroup of patients in the second half of the study. The explanation for this occurrence may be, as previously mentioned, the older age of the population in this subgroup, despite a higher rate of surgical intervention.
In regression analysis, septic shock and peri-annular complications were the independent predictors of in-hospital mortality or need for urgent valvular surgery. These are known predictors and reported in the literature, particularly in Portuguese series from Level III centers.21,23
Independent predictors of long-term mortality were age, chronic kidney disease, and multidrug-resistant microorganism-associated IE. Both age and the presence of chronic kidney disease are known factors of adverse long-term prognosis. The explanation for the association between long-term death and the occurrence of multidrug-resistant microorganism-associated IE is more difficult to establish but may suggest there is greater “frailty” among patients, with more comorbidities associated with contact with healthcare systems and greater prior use of antibiotic therapy.
Even with the major advances in the last decades, IE mortality remains excessively high,47,48 in-hospital mortality lying between 15 and 30%.2 Two recently published multicentric studies, one a previously mentioned Spanish study, with more than 16 000 IE episodes,39 and the other a European, multinational study with data from 41 countries and including 867 patients,49 showed in-hospital mortality of 20.4% and 17.7%, respectively, slightly higher than the present study (14.5%). In Portugal, there are three large series of patients with IE from four level III centers, Centro Hospitalar e Universitário de São João and Centro Hospitalar do Porto,24 Hospital de Santa Marta,21 and Centro Hospitalar Vila Nova de Gaia Espinho.23 The perioperative mortality in these series was 23.8%, 16.3% and 13.1%, respectively. In comparison with the data from the Portuguese series, the in-hospital mortality found in this study overlapped or was slightly lower, which was greatest difference relative to that reported in the series of the two centers in Porto; they had higher mortality. This difference may be due to a referral bias, since patients transferred to referral hospitals are likely to be more severe and have more complications during the course of the disease requiring urgent surgery.
This issue was addressed by two prospective cohort studies that analyzed differences comparing patients with IE who were or were not transferred to Level III centers.50,51 This situation may also explain a higher in-hospital mortality and longer length of stay at high-volume centers, as well as a higher rate of referral to surgery. However, when an adjustment is made for patient risk, it was found that among hospitals with cardiac surgery, the adjusted mortality was lower in centers with a higher volume of IE episodes per year. These results may suggest the creation of IE referral centers in an attempt to decrease the still high in-hospital mortality.
Unfortunately, IE-associated mortality does not end at the patient's discharge from the IE episode. If it is true that the European guidelines for prevention and treatment of IE recommend active surveillance of signs and symptoms of IE recurrence for 12 months after completion of treatment,2 the data on mortality risk among these patients in the short and long-term remain controversial. Some studies report that mortality is highest in the first year and then decreases, although it remains high compared to the general population.52,53 A Swedish study based on the entire population of the country with IE, showed that the increased risk of long-term mortality persists for more than 5 years after the IE episode.54 Additionally, a Dutch retrospective study showed that the 10-year survival rate of patients who received surgical treatment for IE was comparable to that of the general population.55 These data seem to equate the 10-year mortality of the population of patients who had IE to that of the general population, so it is also important to emphasize that this was the case in the population of patients who underwent surgical treatment. Thus, surgical intervention, when indicated, seems to be a key point not only for survival in the in-hospital period, but may also mean that survival could equal that of the general population in the long-term.
Assessing the mortality data from this study, we can conclude that IE survivors maintain an “excess” mortality at least until the 5th year after the IE episode, with a mortality of 38% and 47% at one and five years, respectively. From the 10th year after IE, there seems to be a stabilization in mortality, which in our study remained around 56%. There were no significant differences in mortality between the groups in the two study periods.
Regarding guidance for valve surgery, in this study 34% of patients underwent surgical treatment, which occurred more frequently in the second period of the study. This figure overlapped with that reported by the Spanish multicenter study (23–35%) and the Portuguese series from Level III Centers (36.9% and 38.8%).21,24,39
Regarding IE prophylaxis, both the latest recommendations of the European Society of Cardiology from 20152 and those of the British Society for Antimicrobial Chemotherapy (BSAC) from 200632 and the American Heart Association from 2007,56 recommend IE prophylaxis only for patients at higher risk of severe disease or death from endocarditis57 (mostly patients with congenital disease, especially cyanotic, previous endocarditis and patients with prosthetic valves) undergoing procedures considered high risk (some types of dental procedures). Additionally, the 2008 National Institute for Health and Clinical Excellence recommendations presented an even more radical restriction for IE prophylaxis, suggesting no prophylaxis prior to any type of procedure, even in high risk58 patients. The data on incidence of endocarditis in England show an increase in incidence after the aforementioned 200859 recommendations. In the present study few patients with an indication for prophylaxis according to the current recommendations were identified, given that few patients underwent previous dental procedures. However, several patients had undergone other invasive procedures (genital-urinary, broncho-pulmonary, etc.). Of these, only a few had prophylactic antibiotic therapy associated with the procedure itself and not necessarily with coverage for the main agents of IE. Thus, it was not possible to clarify the existence of a possible cause-effect relationship between prophylaxis and IE development, as well as the possible role of these invasive procedures not covered in current recommendations.
Although this is one of the few studies with long-term follow-up data and that attempts to present a temporal change in the epidemiology of IE, it has limitations. First, those inherent to the fact that it is an observational, single center and retrospective study (several data that may be relevant are missing or incomplete on the clinical files). Then, the fact that we included some patients who had IE over 16 years, which were certainly approached differently by those treating them. On the one hand, over that time, the European IE recommendations themselves changed, not only regarding prophylaxis but also regarding IE treatment. Then, there is the greater availability and evolution of imaging techniques, diagnostic laboratory techniques, and even surgical techniques and implanted prosthetic material. In addition, intensive/postoperative care itself has also undergone changes. Furthermore, some of the patients included had had an IE episode more than 20 years ago, at a time when records were scarce and in hardy copy. Information bias cannot, therefore, be ruled out.
ConclusionsAdvances in diagnosis and treatment of IE have not led to a significant decrease in mortality in recent years. In fact, in the second time period, in-hospital mortality was double that of the previous period, almost achieving statistical significance. In an era in which IE prophylaxis is increasingly restricted and in which the population is increasingly aged, with multiple comorbidities and with more and more intracardiac devices, it is essential to invest in more effective strategies, focusing on the prevention of health system associated infections, reinforcing the general promotion of oral health and judicious use of antibiotic therapy, not forgetting the early identification of the disease and the rapid referral for valvular surgery, when necessary. The creation or reinforcement of high-volume level III referral centers and of an IE heart team may improve disease prognosis.
Conflicts of interestThe authors have no conflicts of interest to declare.