The limited accessibility and the lack of adherence explain, in part, the low proportion of heart failure (HF) patients undergoing exercise-based cardiac rehabilitation (CR) programs. Home-based programs showed to be as effective and less costly than centre-based ones and might address those obstacles. Whether the evidence from international studies can be applied to our population is still unclear.
ObjectivesTo compare the clinical and economic impact of a home-based versus centre-based CR intervention in HF patients.
MethodsThis is a single-center, single-blind, parallel groups, non-inferiority pragmatic randomized control trial. Adult HF patients (n=120) will be randomized to either a centre-based or home-based CR program. In both groups’ patients will participate in a 12-week combined CR program with 2 sessions per week. Exercise training (ExT) protocol consists of a combination of endurance [(at 60%–80% of peak oxygen uptake (VO2peak)] and resistance training (elastic bands). Those allocated to the home-based program will start with 4–5 supervised ExT sessions to familiarize themselves with the training protocol and then will continue the remaining sessions at home. The primary endpoint is the change in VO2peak at the end of the 12-week program. Secondary outcomes include alterations in circulating biomarkers, physical fitness, physical activity, quality of life, diet, psychological wellbeing, dyspnea, and cost-effectiveness analyses.
ResultsPatients are currently being recruited for the study. The study started in November 2019 and data collection is anticipated to be completed by December 2022. This is the first study in Portugal comparing the traditional CR program with a home-based program in HF patients. Our study results will better inform healthcare professionals who care for HF patients regarding CR.
A acessibilidade limitada e a baixa adesão explicam, em parte, a reduzida proporção de doentes com insuficiência cardíaca (IC) que participam em programas de reabilitação cardíaca (RC). Os programas no contexto domiciliário podem contornar alguns desses obstáculos e mostraram-se tão eficazes e menos dispendiosos do que os programas hospitalares. Contudo, ainda não está claro se as evidências de estudos internacionais se podem aplicar à realidade portuguesa.
ObjetivosComparar o impacto clínico e económico de um programa de RC no domicílio versus hospitalar em doentes com IC.
MétodosEste é um ensaio randomizado controlado pragmático de não inferioridade, unicêntrico, de grupos paralelos. Doentes adultos com IC (N=120) serão randomizados para um programa de RC no domicílio ou hospitalar. Em ambos os grupos, os doentes participarão de um programa RC com 12 semanas de duração, com duas sessões de treino por semana. O protocolo de exercício físico consiste numa combinação de treino aeróbio [(a 60%-80% do consumo máximo de oxigênio (VO2pico)] e treino de resistência muscular (bandas elásticas). Os doentes alocados ao programa de RC no domicílio irão realizar quatro a cinco sessões de exercício físico supervisionadas para se familiarizar com o protocolo de treino e depois continuar com as restantes sessões em casa. O outcome primário do estudo é a variação no VO2pico no fim do programa. Os outcomes secundários incluem alterações em biomarcadores plasmáticos, aptidão física, atividade física, qualidade de vida, dieta, bem-estar psicológico, dispneia e custo-efetividade.
ResultadosNeste momento, o estudo está em fase de recrutamento. O estudo iniciou-se em novembro de 2019 e a recolha de dados está prevista para encerrar em dezembro de 2022. Este é o primeiro estudo em Portugal que compara o programa RC tradicional com um programa RC domiciliário em doentes com IC. Esperamos que os resultados deste estudo possam melhor informar as decisões dos profissionais de saúde sobre RC em doentes com IC.
Heart failure (HF) affects approximately 64 million people worldwide, and accounts for an annual health care cost of $31 billion.1 In Portugal, it is currently estimated that 380000 people live with HF and more than 35000 HF hospitalizations take place in our healthcare system every year.2 Despite the existence of a variety of pharmacological and device therapies for HF, patients still have poor long-term prognosis and quality of life (QoL).3 Exercise-based cardiac rehabilitation (CR) could reduce the risk of hospitalization by 30% and is a Class I Level A recommendation for the management of HF.4 However, CR is still an underused treatment in HF patients. In Europe, less than 20% of HF-eligible patients have access to a CR program.5 Several factors related to health services, physicians and patients’ attitudes explain the current low utilization rates. The imbalance between supply (available CR units) and demand (increasing HF prevalence) is an important barrier to deliver this treatment. In addition, adherence is a relevant and well-recognized problem in CR interventions.6 Time and costs associated with the transportation, lack of time and motivation have also a negative impact on CR programs adherence.6
Home-based interventions might help to bypass two of the main barriers: accessibility and adherence. This allows for the treatment of patients living far away from CR units and to extend the usual short-term duration of the centre-based intervention; the flexibility of the home-based interventions is also a factor that may help to improve the levels of CR participation and adherence.7 Home-based CR programs have promisingly demonstrated to increase participation rates and short-term exercise capacity.8 Technological advances made possible and increasingly affordable to monitor at distance the frequency and intensity of exercise; telemonitoring allows the individual tailoring of exercise prescription and distance coaching, as well as enhancing adherence.8,9 Moreover, home-based CR has been reported to be a safe, beneficial9 and cost-effective intervention.10
The heterogeneity of the populations and exercise prescriptions previously studied limits the external validity of available evidence to our national context. Contextual differences such as the type of health system organization and reimbursement policies, and patient characteristics and literacy may significantly influence the clinical effectiveness of a home-based exercise standardized delivery provision. Therefore, further evidence is needed to better inform physicians and health policy makers regarding the beneficial effects and cost-effectiveness of home-based CR programs in Portugal.
This study aims to compare the clinical and economic impact of a home-based versus centre-based CR intervention in HF patients. The powered primary outcome is the change in peak oxygen consumption (VO2peak). Secondary outcomes are alterations in physical fitness, physical activity, QoL, adherence to the Mediterranean diet, psychological wellbeing, dyspnea, selected disease-related biomarkers, and cost-effectiveness indicator. We hypothesised that the home-based intervention would result in a similar improvement in VO2peak and other secondary outcomes and would be cost-effective compared with a centre-based intervention.
MethodsThis study protocol was registered on 6th April 2020 in the database of clinical trials (clinicaltrials.gov) under the registration number NCT04334603 (protocol version 2) (https://clinicaltrials.gov/ct2/show/NCT04334603). Trial registration includes all components of the World Health Organization Trial Registration Data Set, as recommended by the International Committee of Medical Journal Editors. A SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) checklist11.
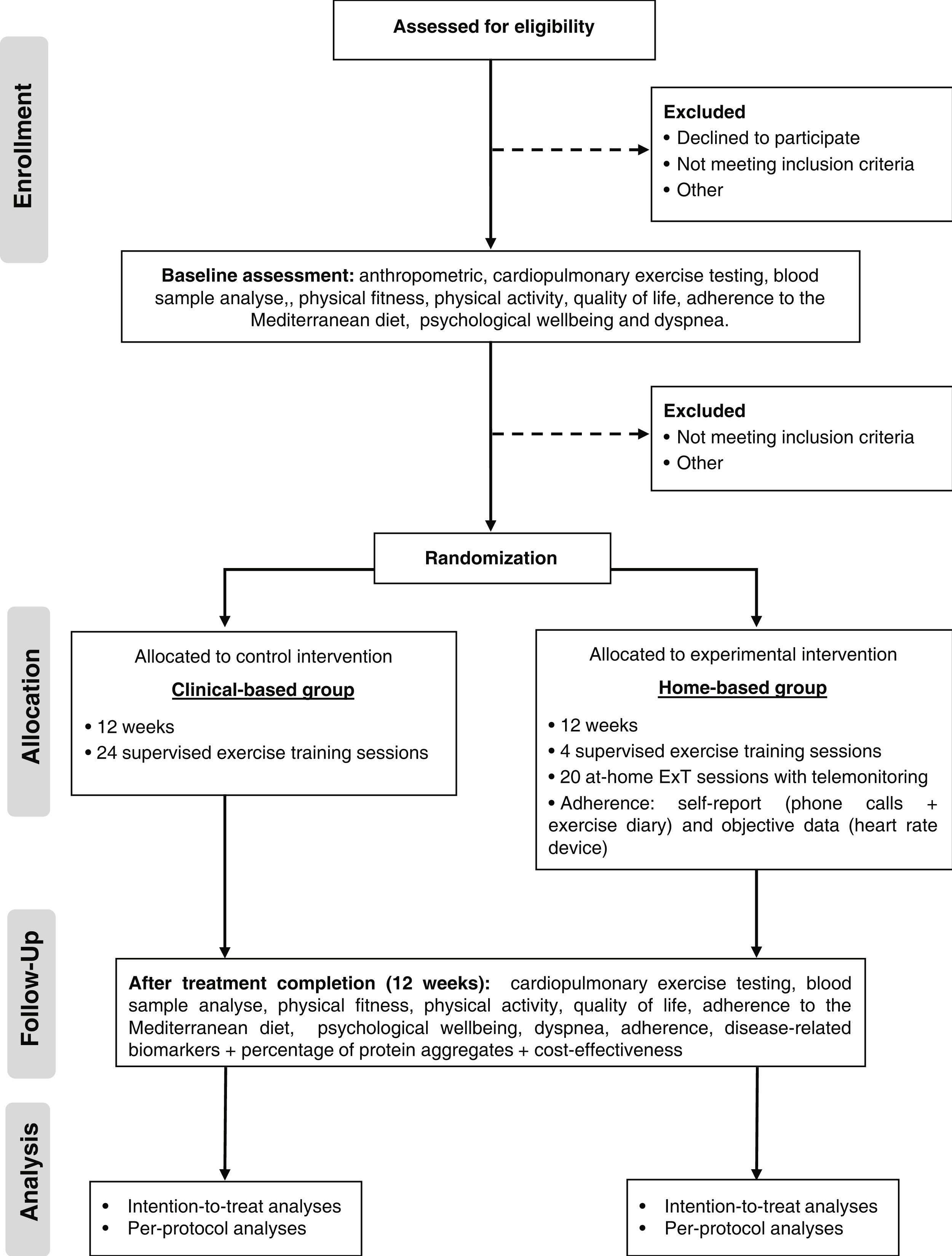
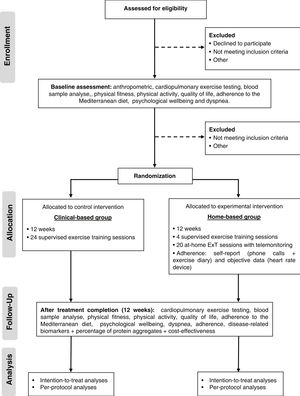
Trial design and settingThe study is a single-center, single-blind, parallel groups, non-inferiority pragmatic randomized controlled trial. Patients with HF will be randomized into two groups: centre-based (standard CR) and home-based CR. Figure 1 shows a schematic representation of the study protocol. The study will be conducted at Centro Hospitalar Universitário de Santo António (CHUdSA), a tertiary university public hospital in Porto, Portugal.
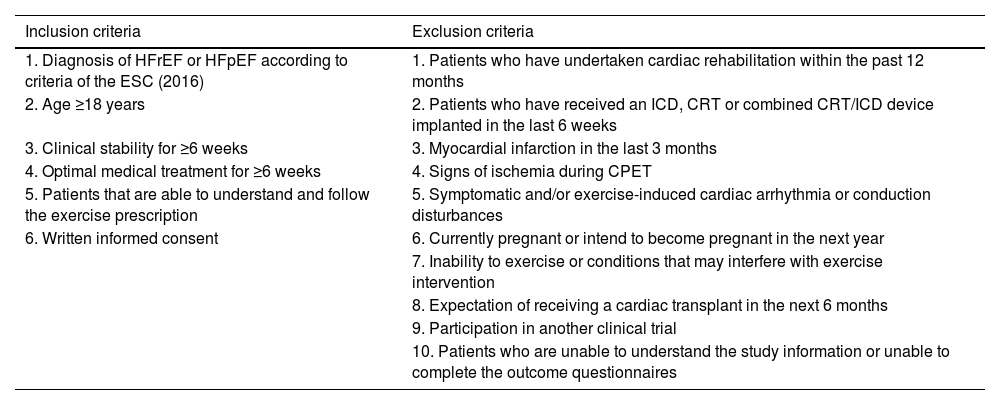
ParticipantsOne hundred and twenty patients (men and women) diagnosed with HF [(HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF)] according to the criteria of the European Society of Cardiology (ESC)4 will be evaluated and randomized. Inclusion and exclusion criteria are listed in Table 1. Individual participants will be discontinued from the trial, in agreement with their physician where appropriate, if any major surgery or health condition arises that will significantly affect their safety to participate in exercise for a duration of >one month, or if the participant elects to withdraw their consent for any reason.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Diagnosis of HFrEF or HFpEF according to criteria of the ESC (2016) | 1. Patients who have undertaken cardiac rehabilitation within the past 12 months |
| 2. Age ≥18 years | 2. Patients who have received an ICD, CRT or combined CRT/ICD device implanted in the last 6 weeks |
| 3. Clinical stability for ≥6 weeks | 3. Myocardial infarction in the last 3 months |
| 4. Optimal medical treatment for ≥6 weeks | 4. Signs of ischemia during CPET |
| 5. Patients that are able to understand and follow the exercise prescription | 5. Symptomatic and/or exercise-induced cardiac arrhythmia or conduction disturbances |
| 6. Written informed consent | 6. Currently pregnant or intend to become pregnant in the next year |
| 7. Inability to exercise or conditions that may interfere with exercise intervention | |
| 8. Expectation of receiving a cardiac transplant in the next 6 months | |
| 9. Participation in another clinical trial | |
| 10. Patients who are unable to understand the study information or unable to complete the outcome questionnaires |
Patients will be consecutively recruited at the Unit of CR of Cardiology Department of Centro Hospitalar Universitário de Santo António (CHUdSA), Portugal between November 2019 and December 2022. Eligible participants will receive the participant information sheet and sign the participant consent form in the first day of evaluations.
RandomizationFollowing the cardiopulmonary exercise testing (CPET), patients will be randomized to a centre-based or home-based CR program. Although we started the study with a randomization ratio of 1:1, we had to adjust to a 1:2 ratio due to the COVID pandemic. The centre-based program required the patient to travel to the hospital and have contact with many people, which put patients at an increased risk of SARS-CoV-2 infection. Therefore, we decided to favour the home-based group to be able to continue the study. To ensure between group balance, it performed a covariate adaptive randomization using the method of minimization. The minimization criteria were HF phenotype (HFrEF vs HFpEF) and baseline VO2peak (<17.5 vs 17.5-24.5 vs >24.5 mL/kg/min). An independent research officer performed the randomization (software minimPy®). The allocation group was communicated to the patients by a researcher after the CPET. Patients were not blinded owing to the nature of the intervention. Except for those delivering the intervention, the staff were blinded to allocation. A blinded researcher conducted the CPET before and after the intervention, and another blind researcher collected the data into a database.
InterventionsThe design of the ExT program follows the exercise recommendations for patients with established chronic HF, regardless of left ventricular ejection fraction.12 In both groups’ patients participate in a 12-week combined ExT program with 2 training sessions per week, for a total of 24 sessions. ExT protocol consists of 5–10 minutes of warm-up with calisthenic and stretching exercise, 25 minutes of resistance exercises using elastic bands with 2 sets of 12–15 repetitions of ten exercises (squat, leg curl, leg abduction, leg adduction, standing calf raise, bench press sitting, seated row, biceps, triceps, lateral raises), 30 minutes of moderate to vigorous aerobic training at 60%–80% of VO2peak (11–14 Borg's scale), and 5 minutes of cool down with stretch exercises. In both groups, patients are encouraged to walk at home at least a third time, a minimum of 30 minutes.
Centre-based groupThe centre-based group will receive a CR program at the hospital, which includes 24 supervised ExT sessions and counselling for lifestyle modification. ExT will be monitored with real-time Ergoline ERS 2, Germany (ECG) and heart rate monitor (model Polar M200; Polar Electro Ltd). During aerobic training, the speed and inclination of the treadmill will be adjusted to ensure that every training session were carried out at the assigned heart rate level. The resistance training intensity will be progressively increased according to rated perceived exertion scale. When patients performed the set comfortably (11–14 Borg's scale) and would be able to perform more two repetitions than the prescribed ones, the intensity will be increased. Adherence, defined as the total exercise sessions during the intervention, will be evaluated by measuring sessions attendance.
Home-based groupThe home-based group participates in a technology-enabled (computer or mobile phone application linked to a wearable smartwatch) program following the same ExT prescription of the centre-based program. The structure of the program is like most trials included in the Cochrane meta-analysis comparing home to centre-based CR13 and consists of unsupervised exercise sessions (walking), weekly phone calls and counselling for lifestyle modification. At the beginning of the program, those allocated to the home-based program will undergo 4–5 supervised ExT sessions (equal to those in the centre-based program) in the CR Unit to familiarize themselves with the training protocol and learn how to use the wearable smartwatch (heart rate monitor, model Polar M200; Polar Electro Ltd, Kempele, Finland) and fill out the exercise logs. Each participant was given a logbook with photographic and written instruction on how to perform and progress each exercise. After these sessions, patients will start training in their home environment (20 exercise sessions). Every exercise session completed by the participant will be recorded by the smartwatch and uploaded to the Polar Flow application (Polar Flow, Polar Electro Ltd, Kempele, Finland). Participants will be telephoned every week to monitor progress. During phone calls, a semi-structured interview will be conducted with the following objectives: to verify adherence to the exercise prescription (crossing self-reported information with data gathered from the heart rate monitor of the smartwatch), to identify problems/barriers to achieving the exercise goals, to provide training-specific advice for the adaptation of the exercise program on the patient's home environment, adjust ExT intensity and accomplish the recommended prescription, and to provide counselling for lifestyle modification. Patients will be instructed to contact the rehabilitation center staff if they experience any symptoms during and after exercising. Adherence will be evaluated by the smartwatch, exercise logs, and telephone.
Nutritional and psychological consultationDuring the CR consultation, all patients that are identified in need of nutrition counselling will be sent to the nutritional consultation (for all patients in general and specifically for diabetic, obese, frail, hypertensive and dyslipidemic patients). In addition, patients will be sent to psychology and/or psychiatry consultations according to the clinical indication.
Socioeconomic supportFinancial support from a social worker will be provided in cases of economic difficulties in obtaining essential medication and transport to the CR center.
AssessmentsDemographic and clinical dataSociodemographic and clinical data (including lipid profile, hemoglobin A1c smoking status, and office blood pressure) will be captured from patients’ files and/or during the CR consult. Clinical files might also be used to complete and/or confirm the patient's profile. The following information will be recorded: age, gender, marital status, educational level, HF diagnosis (etiology, left ventricular ejection fraction, and New York Heart Association functional class), medication and comorbidities. Changes in medication throughout the study will be recorded.
AnthropometricBody height will be measured standing upright against a stadiometer (Seca 213). Weight and body mass index will be measured with patients lightly dressed, using a body composition monitor (Tanita, Inner Scan BC 532). Waist circumference will be measured at the umbilical level.
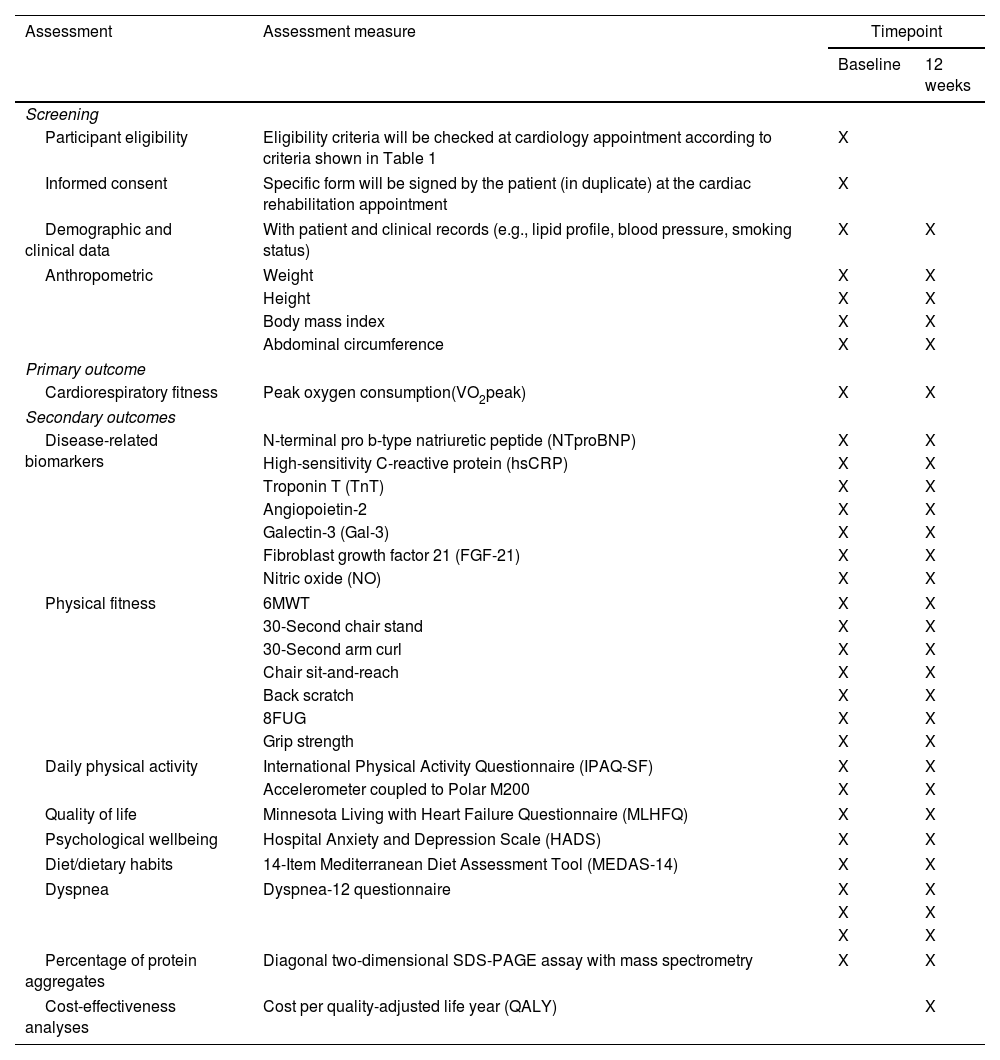
Outcome measurementsAssessment of the outcome's measures is depicted in Table 2. All evaluations will be conducted at CR Unit, at CHUdSA, at baseline and after 12 weeks.
Timeline of assessments.
| Assessment | Assessment measure | Timepoint | |
|---|---|---|---|
| Baseline | 12 weeks | ||
| Screening | |||
| Participant eligibility | Eligibility criteria will be checked at cardiology appointment according to criteria shown in Table 1 | X | |
| Informed consent | Specific form will be signed by the patient (in duplicate) at the cardiac rehabilitation appointment | X | |
| Demographic and clinical data | With patient and clinical records (e.g., lipid profile, blood pressure, smoking status) | X | X |
| Anthropometric | Weight | X | X |
| Height | X | X | |
| Body mass index | X | X | |
| Abdominal circumference | X | X | |
| Primary outcome | |||
| Cardiorespiratory fitness | Peak oxygen consumption(VO2peak) | X | X |
| Secondary outcomes | |||
| Disease-related biomarkers | N-terminal pro b-type natriuretic peptide (NTproBNP) | X | X |
| High-sensitivity C-reactive protein (hsCRP) | X | X | |
| Troponin T (TnT) | X | X | |
| Angiopoietin-2 | X | X | |
| Galectin-3 (Gal-3) | X | X | |
| Fibroblast growth factor 21 (FGF-21) | X | X | |
| Nitric oxide (NO) | X | X | |
| Physical fitness | 6MWT | X | X |
| 30-Second chair stand | X | X | |
| 30-Second arm curl | X | X | |
| Chair sit-and-reach | X | X | |
| Back scratch | X | X | |
| 8FUG | X | X | |
| Grip strength | X | X | |
| Daily physical activity | International Physical Activity Questionnaire (IPAQ-SF) | X | X |
| Accelerometer coupled to Polar M200 | X | X | |
| Quality of life | Minnesota Living with Heart Failure Questionnaire (MLHFQ) | X | X |
| Psychological wellbeing | Hospital Anxiety and Depression Scale (HADS) | X | X |
| Diet/dietary habits | 14-Item Mediterranean Diet Assessment Tool (MEDAS-14) | X | X |
| Dyspnea | Dyspnea-12 questionnaire | X | X |
| X | X | ||
| X | X | ||
| Percentage of protein aggregates | Diagonal two-dimensional SDS-PAGE assay with mass spectrometry | X | X |
| Cost-effectiveness analyses | Cost per quality-adjusted life year (QALY) | X | |
The primary outcome of the study is the change in VO2peak after 12 weeks. VO2peak is the gold standard for assessing cardiovascular fitness and is a strong prognostic indicator in HF patients.14 VO2peak will be evaluated through the maximal CPET testing on a treadmill (Medisoft, Model 870C). The testing protocol will be chosen according to the patient's physical activity (PA) level and orthopedic/musculoskeletal condition (modified Naughton or Bruce). The CPET will be conducted under medical supervision, with electrocardiography continually monitored throughout the protocol. Using a stationary metabolic cart system (Geratherm® Respiratory Ergostik, under BLUE CHERRY®), respiratory gas exchange measurements will be obtained breath-by-breath and recorded every 30 seconds. Heart rate and blood pressure will be recorded at regular intervals throughout the test. During CPET testing, patients will be strongly encouraged to achieve a Borg rating of perceived exertion score >17 on the 6 to 20 scale, and a respiratory exchange ratio >1.10. Peak VO2 will be determined as the highest VO2 achieved during exercise.
Secondary outcomesDisease-related biomarkersAll subjects will undergo venous blood sampling drawn from an antecubital vein at baseline and after 12 weeks. Alterations in disease-related biomarkers, such as N-terminal pro b-type natriuretic peptide (NTproBNP), high-sensitivity C-reactive protein (hsCRP) and Troponin T (TnT) will be assessed using the standard protocol of our hospital laboratory. The circulating levels of other important functional biomarkers in HF, including galectin-3 (Gal-3), fibroblast growth factor 21 (FGF-21), and angiopoietin-2 (Ang2), will be quantified using commercially available ELISA kits. Nitric oxide (NO) will be indirectly measured by the quantification of nitrate and nitrite using a total NO assay kit. All the measurements will be performed in duplicate according to the respective manufacturer's instructions.
NTproBNP has great prognostic significance in these patients.4 hsCRP is a nonspecific marker of the inflammatory response; in HF patients, higher levels are associated with a worst outcome.15 Elevation in TnT is a predictor of adverse outcomes in hospitalized as well as stable ambulatory patients with HF.16 Gal-3 can be used as a prognostic biomarker to monitor the progression and risk of mortality in chronic HF patients.17 Despite the mechanisms underlying the effects of FGF-21 in HF are not fully understood, it has been shown that FGF-21 levels are elevated in HF, and have good predictive values for adverse cardiac events within 1 year.18 Ang2 had been mostly associated with acute cardiovascular events.19 However, higher levels of Ang2 in serum were also seen in chronic HF patients of increasing severity.20 In HF, endothelial function is compromised due to higher levels of inflammation and oxidative stress, which will impair NO production.21
Physical fitnessChanges in physical fitness levels will be evaluated by the Senior Fitness Test battery.22 Physical fitness in HF patients is related with better QoL and, an improvement in physical fitness may be associated with a reduction in cardiac rehospitalization and all-cause mortality.23
Six-minute walking test (6MWT): it will be performed in an indoor corridor with a course of 25 m, marked every 5 m with cones. Baseline oxygen saturation, heart rate, brachial arterial blood pressure and the Borg scale rating will be recorded. During the test, the participants must walk as fast as they can, and they are allowed to stop or slow down if they feel like doing it. At the end of the test, the Borg scale for dyspnea and fatigue and heart rate will be measured. The number of laps and the additional distance covered will be recorded.
30-Second chair stand: this test assesses lower body strength. To perform the test, patients will be required to stand up and sit down quickly and as safely as possible. The number of repetitions performed in 30 seconds, with the arms crossed over the chest, will be registered.
30-Second arm curl: this test assesses upper body strength. Sitting in a chair, the patient should flex the forearm with a 2.27 kg weight for women and 3.63 kg for men for 30 seconds. The number of repetitions performed during the 30s will be registered.
Chair sit-and-reach: this test assesses lower body flexibility. From a sitting position in front of a chair, the patient tries to reach the toes with the leg straight in the knee joint. The distance between extended fingers and the tip of the toe (+ or −), in centimeters, will be recorded and the best value will be taken for analysis.
Back scratch: this test assesses upper body (shoulder) flexibility. The patient tries to join the hands behind the back, leading one hand from the top, and the other from the bottom. The result is the distance between the middle fingers (+ or −), in centimeters. The best value of two repetitions will be taken for analysis.
Eight-foot up-and-go test (8FUG): the test assesses agility and dynamic balance. The patient starts the test in a sitting position. After a signal, the patient must stand up, walk 2.44 meters, make a turn around a cone, and return to the initial position as fast as possible. The patients will perform the test twice and the best time will be considered.
Handgrip strength: strength (kg) will be measured with an isometric hand dynamometer (Lafayette Model 78010, 78011, Indiana, USA). Both arms will be measured 3 times while patients were seated, with the shoulder adducted and neutrally rotated, the elbow flexed at 90̊, and the forearm and wrist in a neutral position. The best attempt will be used as the final score.
Daily physical activityPhysical activity will be measured through a questionnaire and a triaxial accelerometer copulate to the Polar M200. Self-reported PA will be assessed with the short form of the International Physical Activity Questionnaire (IPAQ-SF)24 through a personal interview to identify time spent in specific behaviours. Total weekly PA in METs will be estimated using the instrument's scoring protocol (3.3 METs to walking, 4.0 METs to moderate, and 8.0 METs to vigorous activity).
PA will be evaluated objectively using the Polar M200 (Polar Electro Ltd) to measure time spent in light, moderate and vigorous physical activity, time spent sitting and lying, and the number of daily steps. Minutes spent in each activity type will be summed to a total day score. Patients will be instructed to wear the watch device during 24 hours for a period of 7 consecutive days.
Quality of lifeChanges in QoL will be evaluated through the Minnesota Living with HF Questionnaire (MLHFQ),25 a disease-specific questionnaire for patients with HF. The MLHFQ encompasses 21 questions, whose purpose is to determine how the disease affects the physical, psychological, and socioeconomic conditions of the patients during the previous month. The MLHFQ total score range from 0 to 105 (no impairment to maximum impairment). Higher MLHFQ score means worse QoL. Two other scores can be determined: the physical dimension (8 items, 0–40), and the emotional dimension (5 items, 0–25). Answers options range from 0 (none) to 5 (very much), where 0 represented no limitation and 105 represented maximal limitation.
Psychological wellbeingThis will be evaluated by the Hospital Anxiety and Depression Scale (HADS).26 The questionnaire is composed of 14 questions on a four-point (0–3) scale. The possible scores ranged from 0 to 21 for anxiety and 0 to 21 for depression. A score of 0–7 for either subscale will be regarded as being in the normal range, a score of 11 or higher indicates the probable presence of the mood disorder and a score of 8–10 is just suggestive of the presence of the respective state.
Diet/dietary habitsThe dietary habits will be evaluated by the 14-Item Mediterranean Diet Assessment Tool (MEDAS-14) questionnaire.27 The answer to each of the 14 items is scored with 1 in the case of meeting the criteria defined as typical of this type of food (range of possible variation 0–14 points). A total score ≥10 represents a good adherence to the Mediterranean diet.
DyspneaDyspnea symptoms will be evaluated by the Dyspnea-12 (D-12) questionnaire.28 It provides an overall score for breathlessness severity that incorporates seven physical items and five affective items. The D-12 consists of 12 descriptor items on a scale of none (0), mild (1), moderate (2), or severe (3) symptoms. Total scores from the D-12 range from 0 to 36, with higher scores corresponding to greater severity.
Percentage of protein aggregatesProtein aggregates will be analysed using a diagonal two-dimensional (D2D) SDS-PAGE assay with mass spectrometry to identify and characterize detergent-resistant protein aggregates in plasma pre-cleared from albumin and immunoglobulin in a subgroup of 20 patients. Protein homeostasis (proteostasis) is frequently impaired in HF. The overload of protein degradation pathways and collapse of protein quality control will halt protein turnover and is associated with the formation of toxic protein aggregates and misfolded/dysfunctional proteins that contribute to the pathophysiology of the disease.29
Cost-effectiveness analysisTo compare the cost-effectiveness of centre-based versus home-based CR intervention, we will use a 5-dimension questionnaire, the EQ-5D, to measure health-related QoL. We will differentiate between cardiac health care costs, noncardiac health care costs, and non-health care costs. The following information related with the cost of the resources used will be collected: type and the number of visits (urgent and non-urgent visits), procedures and medication associated to the visits and length of stay in case of hospitalization. A survey will be conducted to estimate the patients travel time and costs, work status, hours of absenteeism from work, and the time that each health professional spends with the patients during the CR program. We intend to calculate the costs from a societal perspective, which include medical direct costs, non-medical direct costs, and indirect costs, mainly those related with losses in productivity. We will estimate the incremental cost-effectiveness ratio, that is, ratio between the difference of average costs for home-based exercise programs and the control exercise approach program and the difference between average quality-adjusted life years (QALYs) of the two exercise programs.
Data collection, management, and monitoringThe collection, storage, and use of data for the research purpose will be explicitly authorize by patients in the signed consent form. All documents will be stored securely in confidential conditions and will only be accessible only by the study investigators. Patients will be identified at database and project specific documents by the study participant number. A cloud-based platform will be designed and open to all project members, to centralize data into a repository. All hard-copy files will be stored in a securely locked filing cabinet.
Research fundings will be disseminated through peer reviewed journals with impact to the scientific community, and public presentations (to clinicians, to academic audiences, and in national and international meetings).
Study-related monitoring, audits, and inspections of all study-related documents and facilities by the hospital ethic committee will be permit. Given the role of the hospital Ethical Authorities, an independent data monitoring committee will be not needed.
Ethics and disseminationThe study design conforms with the principles outlined in the Declaration of Helsinki and National Legislation, according to the Good Clinical Practices guidelines and respecting patients’ confidentiality. The study was approved by the local research Ethics Committee [2019/123(103-DEFI/107-CE)].
Statistical analysesSample size calculation was performed for the primary endpoint: change in VO2peak at 12 weeks. Based on a previous trial30 and to accommodate a 10% attrition rate, 120 patients (60 per group) are required to test the hypothesis that the home-based intervention is not inferior to the centre-based one, assuming a non-inferiority limit of 1.25 mL/kg/min at the 2.5% significance level and 80% power.
The normality of data distribution will be evaluated with the Shapiro–Wilk test or by kurtosis and skewness test. Baseline patient characteristics will be summarized using the mean and standard deviation or median and interquartile ranges for continuous variables, as appropriate, and frequencies and percentages for categorical variables. Mean differences will be expressed with their two-sided 95% confidence interval. Between group differences at baseline and in the change from baseline to the end of the intervention will be tested with unpaired Student t tests or Mann–Whitney U test. Analysis of covariance will be also employed to adjust for baseline differences between groups. Within-group comparisons from baseline to the end of the intervention will be analysed using the paired Student t tests or Wilcoxon signed-rank test. Between group comparisons at baseline in categorical variables will be tested with the chi-square test. All statistical analyses will be performed considering the intention-to-treat analysis (using an 80% cut-point for adherence) and a per protocol basis to mitigate bias. Patients with missing values of primary outcome (VO2peak), will be exclude from the analyses. A two-sided p-value <0.05 will be considered significant. There are no interim analyses planned or early trial termination guidelines.
DiscussionThis study will investigate the clinical and economic impact of a home-based versus centre-based CR intervention in HF patients. Various studies performed in other countries showed that home-based CR programs are a safe and beneficial alternative to centre-based CR, and they also tend to be a cost-effective intervention compared with traditional exercise programs.9,10 However, the differences in ecological characteristics such as the type of health system organization and reimbursement policies, and patient characteristics and literacy may significantly influence the clinical effectiveness of a home-based CR.
To test the hypothesis that the home-based CR intervention would result in a benefit like centre-based intervention, and if it can be a cost-effective intervention in the Portuguese healthcare setting, we designed a pragmatic clinical trial. Pragmatic trials can test the same intervention as an explanatory trial, but they are conducted in real-world clinical practice settings, with typical patients and by qualified clinicians. For example, we will include patients with peripheral arterial disease, atrial fibrillation, cardiac implanted devices, and older patients, who are generally excluded from the CR studies.
To our knowledge, this is the first study performed in Portugal with HF patients, that will study a home-based CR program using accessible and low-cost technology. We expect that this study can contribute to increasing the awareness of the importance of CR in HF and to provide data on different modalities of delivering CR. Each of these options should be considered in the local practice healthcare unit and to the individual HF patient to address common barriers to delivering CR such as lack of resources, and geographical and travelling constraints.
This is the first pragmatic randomized clinical trial in Portugal comparing the traditional CR program with a home-based program in HF patients. Our study results will contribute to provide better information to healthcare professionals and hospitals who care for HF patients and to policy makers deciding on reimbursement policy regarding CR.
Authors’ contributionsStudy concept and design: CS, FR and MS; Acquisition, analysis, or interpretation of data: CS, SM, PGB, MG, MT, CS, AIT, FR and MS; Drafting of the manuscript: CS, FR, JPF and MS; Critical revision of the manuscript: CS, SM, PGB, MG, MT, CS, AIT, FR and MS. All authors have read and agreed to the published version of the manuscript.
FundingThis work was financially supported by the project POCI-01-0145-FEDER-030011, funded by , through COMPETE2020-POCI, and by national funds, through FCT/MCTES (PTDC/MEC-CAR/30011/2017).
Conflicts of interestThe authors declare no conflicts of interest.