Coronary artery disease is the most important cause of late morbidity and mortality after heart transplantation. It is usually an immunologic phenomenon termed cardiac allograft vasculopathy, but can also be the result of donor-transmitted atherosclerosis. Routine surveillance by coronary angiography should be complemented by intracoronary imaging, in order to determine the nature of the coronary lesions, and also by assessment of their functional significance to guide the decision whether to perform percutaneous coronary intervention. We report a case of coronary angiography at five-year follow-up after transplantation, using optical coherence tomography and fractional flow reserve to assess and optimize treatment of coronary disease in this challenging population.
Doença coronária é a causa mais importante de morbimortalidade tardia após transplantação cardíaca. Habitualmente, representa um fenómeno imunológico designado como vasculopatia do aloenxerto, mas também pode resultar da aterosclerose transmitida pelo dador. A vigilância através de coronariografia de rotina deve ser complementada pela utilização de imagem intracoronária, de forma a determinar a natureza das lesões, e também através de uma avaliação funcional para tomada da decisão de realizar intervenção coronária percutânea. Apresentamos o caso de uma coronariografia aos cinco anos de seguimento após transplantação, utilizando tomografia de coerência ótica e fractional flow reserve para avaliação e otimização do tratamento da doença coronária nesta população desafiante.
bare-metal stents
cardiac allograft vasculopathy
drug-eluting stents
fractional flow reserve
intravascular ultrasound
left anterior descending artery
left internal mammary artery graft
optical coherence tomography
orthotopic heart transplantation
percutaneous coronary intervention
Orthotopic heart transplantation (OHT) is the current mainstay treatment for end-stage heart failure refractory to other therapies.1 Heart transplant coronary artery disease is largely an immunologic phenomenon termed cardiac allograft vasculopathy (CAV), and is the most significant cause of late morbidity and mortality after OHT.2–4 There is an exponential growth in the incidence of CAV after five years following transplantation, and some studies have also shown an approximately 10% increase in disease incidence with every two-year interval after OHT.5
Typically, cardiac transplant recipients do not experience angina because of perioperative denervation. However, they may present with left ventricular dysfunction as a consequence of myocardial ischemia. Therefore, routine surveillance for CAV is recommended by the International Society for Heart and Lung Transplantation, for which coronary angiography is considered the modality of choice.3
Treatment of CAV remains a clinical challenge. The options are limited and include the use of oral antiproliferative agents, statins, percutaneous coronary intervention (PCI) and/or repeat OHT, all with suboptimal results.6
Case reportWe present the case of a 64-year-old male with ischemic cardiomyopathy who received an OHT from a 42-year-old male donor in 2009. The patient had three-vessel disease and had undergone coronary artery bypass grafting in 1998; in the pre-heart transplantation evaluation only the left internal mammary artery (LIMA) to left anterior descending artery (LAD) graft was patent. Two pre-existing non-occlusive (<50%) stenoses in the mid LAD of the donor heart led to the decision to use the patient's LIMA to anastomose to the donor's LAD during the transplantation procedure.
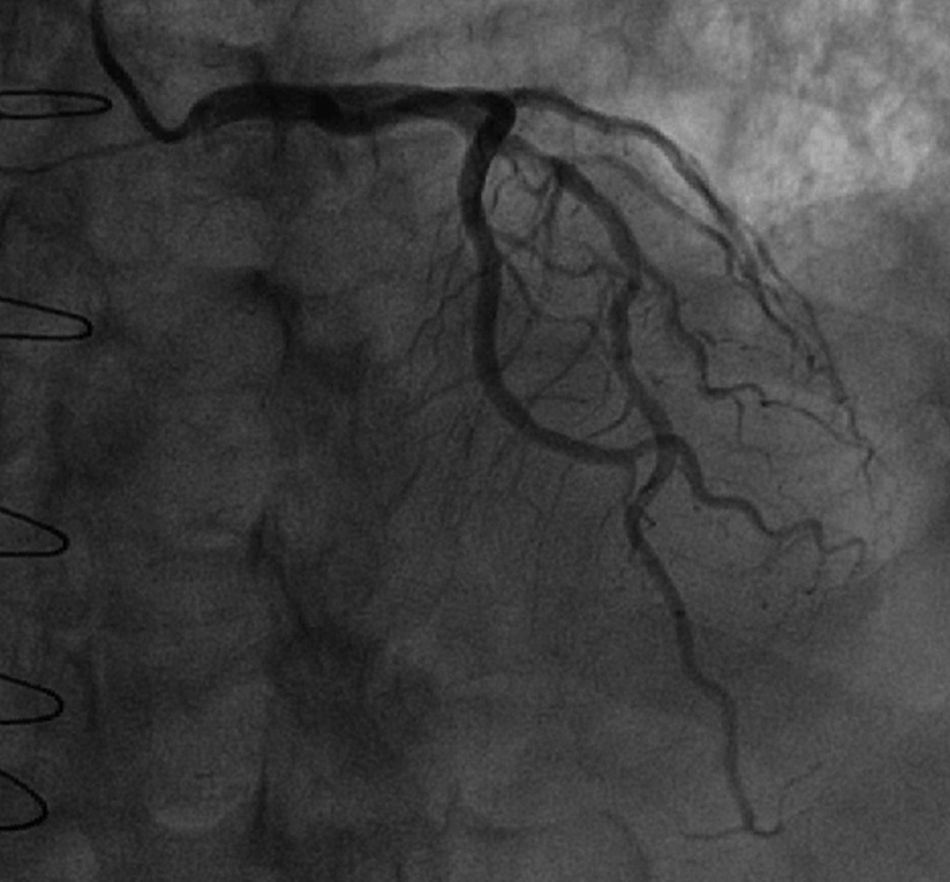
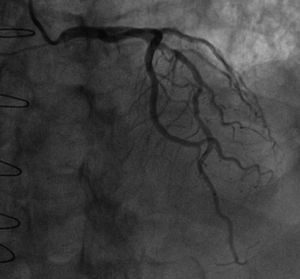
At five-year follow-up after transplantation, the routine coronary angiography (Figure 1) showed progression of the two mid LAD stenoses (50-70%) and revealed that the LIMA–LAD graft was no longer patent.
The functional significance of the LAD lesions was assessed by measurement of fractional flow reserve (FFR) using a 0.014″ pressure guide wire (PressureWire Aeris™, St. Jude Medical, Uppsala, Sweden). The value obtained was 0.78, suggesting the presence of functionally significant lesions.
The pressure guide wire was then replaced by a 2.7F optical coherence tomography (OCT) catheter (Dragonfly Duo™ imaging catheter, St. Jude Medical, St. Paul, MN, USA) and OCT images (Ilumien Optis™ system, St. Jude Medical, St. Paul, MN, USA) were recorded from the mid to proximal portions of the LAD at an automatic pull-back speed of 20 mm/s and a frame rate of 100/s.
OCT images of the mid LAD segment (Figure 2) showed a lipid-rich plaque with atherosclerotic characteristics, and OCT of the proximal LAD segment (Figure 3) demonstrated intimal hyperproliferation compatible with CAV.
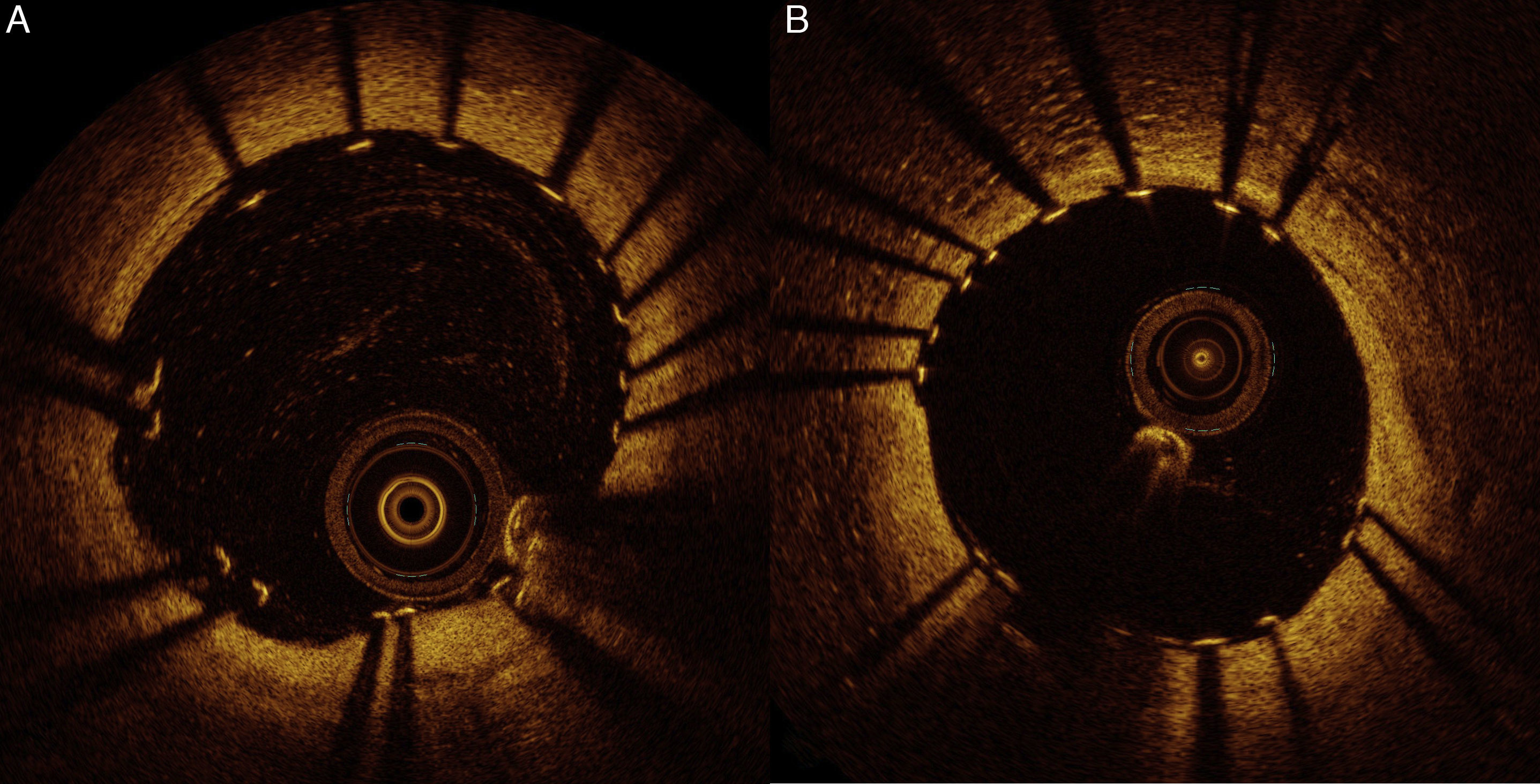
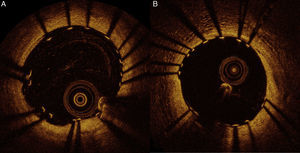
Considering these findings, we decided to treat the two lesions with 3.0 mm×15 mm and 3.0 mm×12 mm Ultimaster™ sirolimus-eluting stents. The result was assessed by OCT and revealed malapposed struts of the distal stent (maximum length of segments with malapposed struts 1.5 mm; maximum malapposition distance 0.36 mm). We therefore decided to proceed with adjunctive post-dilatation using a 3.0 mm×12 mm non-compliant balloon. Final OCT examination showed a very good result, as presented in Figure 4. FFR was repeated, indicating a good physiological result of 0.89.
DiscussionHeart transplant coronary artery disease represents a unique clinical challenge, due to its distinctive pathogenesis, diagnostic approach and treatment modalities. CAV is thought to be an immunologic phenomenon that involves the entire coronary vasculature diffusely, with marked intimal proliferation and concentric vascular thickening and fibrosis.7 This contrasts with conventional coronary atherosclerosis, which is marked by focal, eccentric fibrofatty atheroma.7
Advances in invasive coronary imaging, such as intravascular ultrasound (IVUS) and OCT, have shown promising results in increasing knowledge of the pathophysiology of CAV. Intimal thickening is typically the initial finding on IVUS, and rapidly progressive CAV is defined as an increase of ≥0.5 mm in intimal thickness within the first year post-OHT.8 More recent studies using intracoronary OCT have demonstrated higher sensitivity for early detection of CAV,9 and have provided new insights that extend beyond the traditional concept of CAV, showing the development of atherosclerosis with vulnerable plaque and complicated coronary lesions such as intraluminal thrombus.10 Although both IVUS and OCT are more sensitive and specific in detecting early-stage CAV than coronary angiography alone and provide additional information for risk stratification,11 routine use of these methods is limited. The current recommendation for screening and surveillance of CAV remains annual coronary angiography. Nevertheless, IVUS with a baseline study at 4-6 weeks and at one year after OHT, in order to exclude donor coronary artery disease and to detect rapidly progressive CAV, is already mentioned as an optional method.12
Coronary artery disease in heart transplant recipients is often a combination of donor-transmitted atherosclerosis and de novo lesions of CAV. This distinction can be difficult to make solely on the basis of the distribution and morphology of lesions on coronary angiography. Though the lesions of CAV tend to be more diffuse and circumferential than donor-transmitted atherosclerosis, both types of lesions have a predilection for proximal segments and bifurcation sites.13 In the case reported, the progression of pre-existing non-obstructive stenoses in the mid LAD segment of the donor heart was easily detected by angiography and their nature confirmed by OCT imaging.
The etiology of the heart failure that leads to OHT may also be an important factor in the progression of transplant coronary artery disease. Guddeti et al.14 demonstrated that ischemic cardiomyopathy patients had significantly greater plaque volume index at initial and follow-up virtual histology IVUS examinations and that this was an independent predictor of plaque progression, especially within three years of transplantation.
Treatment of CAV is truly a challenge since its pathophysiology is multifactorial, with a wide range of immunologic and non-immunologic contributors. Furthermore, patients are usually asymptomatic, but their survival after documentation of CAV is significantly reduced.15 Current immunosuppressive therapy has limited efficacy in preventing the development of CAV.16 By contrast, statins exert multiple pleiotropic effects in the transplanted heart, reducing cytokine activity, improving coronary endothelial function and increasing coronary flow reserve,17 and several statins have been associated with reduced CAV progression and improved survival after OHT.18–20 Repeat OHT is the only definitive therapy for CAV but it is associated with higher perioperative mortality, lower long-term survival and a higher recurrence of CAV.21 Coronary artery bypass surgery is rarely performed, because CAV is associated with poor distal targets, due to the diffuse nature of the disease, and greater perioperative mortality.22,23
Hence, PCI has been routinely used as a treatment alternative in patients with focal proximal CAV.6 Although small-scale, single-center studies have demonstrated excellent procedure outcomes,24,25 data on long-term mortality benefit are sparse and controversial. Recently, Agarwal et al.6 showed that among patients with at least moderate CAV on any coronary angiogram, long-term survival in those amenable to PCI was greater than in those with CAV not treatable with PCI.
The assessment of cardiac transplant recipients by routine coronary angiography entails interpreting the physiological significance of focal coronary stenosis, particularly of intermediate stenosis, in order to guide the need for PCI. FFR is an index that provides information on the degree to which epicardial artery disease is affecting myocardial perfusion and is well validated in non-OHT populations.26 Studies27,28 on the changes in coronary anatomy and physiology after heart transplantation found that soon after OHT there is a strong correlation between FFR and IVUS-determined epicardial percentage plaque volume. Late after OHT, increased microvascular resistance (as shown by a higher index of microvascular resistance) results in a decrease in the maximum achievable flow down the epicardial vessel and lessens the physiologic impact of structural abnormalities in the epicardial artery. For this reason, when significant microvascular dysfunction is present, FFR may not provide a good representation of epicardial artery plaque burden. In this case report, FFR was very useful for assessing the functional significance of the two mid LAD intermediate stenoses, guiding the decision whether to intervene. After PCI an increase in FFR was also demonstrated, proving the physiologic benefit of the PCI.
Finally, PCI in heart transplant recipients is associated with a higher incidence of restenosis, compared to the non-OHT population. Outcomes with balloon angioplasty have been less than ideal, contributing to allograft loss because of the very high incidence of restenosis.29 PCI with drug-eluting stents (DES) appears to be associated with reduced angiographic in-stent restenosis rate in transplant patients.30 Tremmel et al.31 compared PCI with bare-metal stents (BMS) vs. DES in cardiac transplant recipients and demonstrated that DES are safer and more effective in suppressing neointimal hyperplasia after PCI for CAV. This leads to a significantly lower rate of late lumen loss and target lesion revascularization, as well as to reduced rates of cardiac death and nonfatal myocardial infarction. In the case presented, we used two new-generation DES and then assessed the result by OCT imaging. As the distal stent had an incomplete expansion, which is known to be associated with an even higher restenosis rate,32 we performed a high-pressure post-dilation to obtain optimal stent expansion, confirmed by another OCT examination.
ConclusionsHeart transplant coronary artery disease is a complex disorder and remains a significant cause of morbidity and mortality in OHT patients. Invasive coronary imaging modalities such as IVUS or OCT are crucial as they provide information on early-stage CAV and can confirm the nature of coronary stenosis and help in PCI optimization. Although it is affected by microvascular dysfunction in the late stages after OHT, assessment through FFR may be valuable in making therapeutic decisions. Compared with BMS, PCI with DES is safer and reduces the restenosis rate in patients with CAV. The present case demonstrates the use of a multimodality approach and highlights its importance to achieve good outcomes in this challenging population.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.




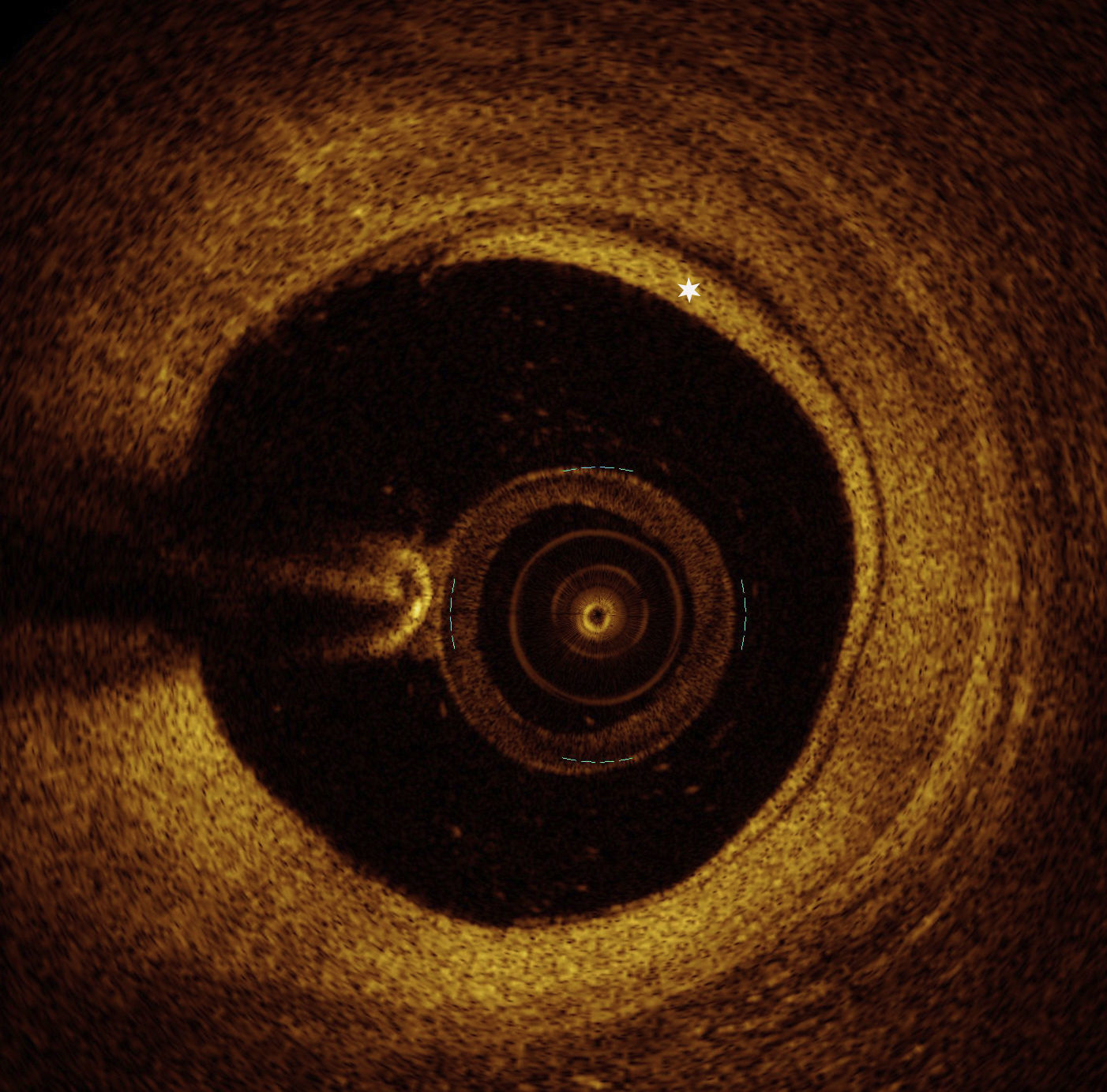
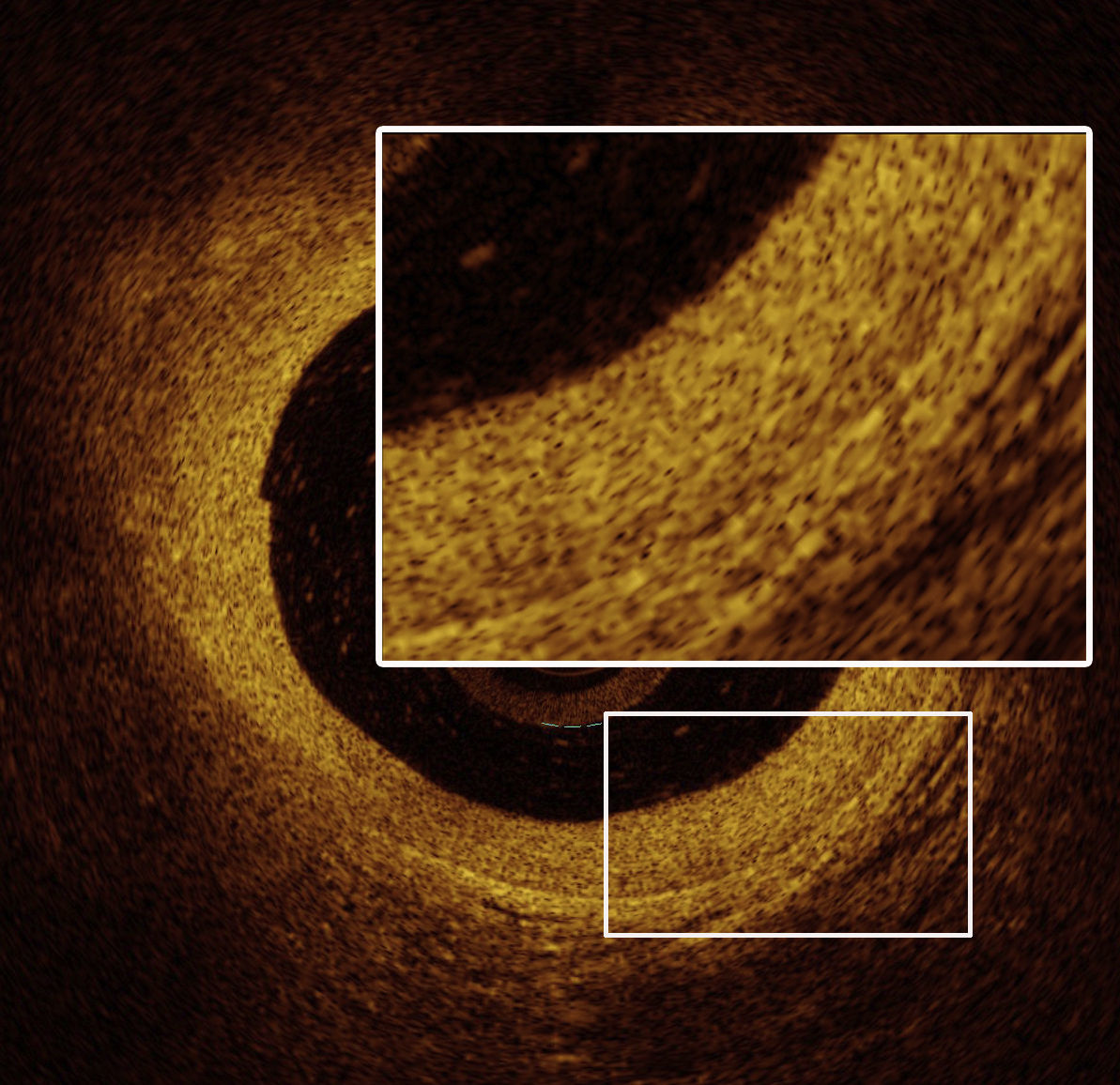
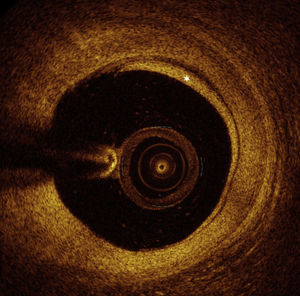
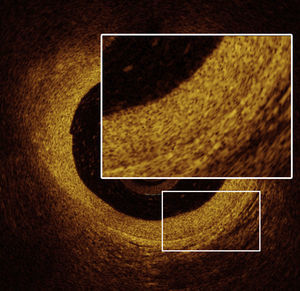
 OCT): image showing distal mid left anterior descending artery (
OCT): image showing distal mid left anterior descending artery (
