Congenital coronary artery anomalies are abnormalities in the origin, course or structure of these arteries, and their incidence varies from 0.2% to 5.6%. Although the majority of anomalies are asymptomatic, they are the second most common cause of sudden cardiac death in young athletes.
The aim of this study is to highlight the main anomalies with hemodynamic significance, particularly anomalous aortic origin of coronary arteries and anomalous left coronary artery from the pulmonary artery.
Anomalous aortic origins of coronary arteries account for 14–17% of all sudden cardiac deaths that unexpectedly occur in healthy children or young athletes during or immediately after physical exercise. The mechanism responsible for the compression/occlusion of the coronary artery originating from the opposite sinus is still unclear and several mechanisms have been proposed. The clinical presentation of these patients varies and physical examination is normal in most cases. Transthoracic echocardiography is the most commonly used test for diagnosis. The treatment, management and follow-up of these patients are the subject of debate.
The anomalous origin of the left coronary artery arising from the pulmonary artery is an even rarer condition and, without corrective surgery, most patients die within the first year of life. Echocardiography is also the method of choice to confirm this condition. The diagnosis of this anomaly in a seriously ill child is an indication for urgent surgery.
Due to the hemodynamic abnormalities caused by these abnormalities, early diagnosis and treatment are important.
As anomalias congénitas das artérias coronárias são alterações da sua origem, trajeto ou estrutura e a sua incidência varia entre 0,2 e 5,6%. Apesar de a maioria ser assintomática, são a segunda causa morte súbita de origem cardíaca em jovens atletas.
O objetivo deste trabalho é salientar as principais anomalias com repercussões hemodinâmicas, nomeadamente as anomalias da origem das artérias coronárias na aorta e as anomalias em que a artéria coronária esquerda tem origem na artéria pulmonar.
As anomalias da origem das artérias coronárias na aorta correspondem a 14-17% de todas as mortes súbitas de causa cardíaca, que ocorrem inesperadamente em crianças saudáveis ou jovens atletas durante ou imediatamente após o exercício físico. O mecanismo responsável pela compressão/oclusão da artéria coronária com origem no seio coronário oposto ainda não é claro e existem vários mecanismos propostos. A apresentação clínica desses doentes é bastante variável e o exame físico é na maioria das vezes normal. O ecocardiograma transtorácico é o exame mais usado para o diagnóstico. A abordagem, o tratamento e o seguimento desses doentes é ainda um tema controverso.
A origem anómala da artéria coronária esquerda na artéria pulmonar é ainda mais rara e a maioria dos doentes morre no primeiro ano de vida, se não for feita correção cirúrgica. O ecocardiograma é também o método de eleição para a sua confirmação. O diagnóstico dessa anomalia numa criança, gravemente doente, é indicação cirúrgica de urgência.
Pelas alterações hemodinâmicas que acarretam, o seu diagnóstico e tratamento precoce são importantes.
Congenital coronary artery anomalies (CCAAs) were first described two millennia ago by Galen and Vesalius1 and are abnormalities in the origin, structure, and course of these arteries.2
In fact, this topic has become increasingly well-known due to its association with sudden death,3 even though most CCAAs are benign, with no hemodynamic or prognostic implications.4
Coronary artery disease is one of the leading causes of morbidity and mortality worldwide. Among these, CCAAs, although less prevalent, are a potential source of malignant arrhythmias, ischemia and myocardial dysfunction.3 In young athletes, CCAAs are the second most common cause of sudden cardiac death (in 12% of deaths), and are generally triggered by vigorous physical exercise.4,5 Sudden cardiac death can be defined as an unexpected death that is cardiac in origin. It generally occurs less than one hour after the onset of symptoms in an individual with no previously known fatal condition.4 According to a study based on the Minneapolis Heart Institute Foundation's records, the two most common causes of sudden death were hypertrophic cardiomyopathy at 36% and CCAAs at 17%.6
Although most CCAAs are asymptomatic and involve no hemodynamic complications, some CCAAs are clinically significant.7 The latter are classified into two subgroups: anomalous origin of coronary arteries from the opposite sinus (left coronary artery originating from the right coronary sinus and right coronary artery originating from the left coronary sinus) and anomalous left coronary artery from the pulmonary artery (ALCAPA).3 These anomalies are associated with sudden death. However, there are other less common CCAAs that may also be related to sudden cardiac death. These include a single coronary artery with an interarterial course, atresia of the coronary ostium or artery fistulas.4,6,8
In the presence of CCAAs, sudden death is related to myocardial ischemia resulting in malignant ventricular arrhythmias. The physiological demands of physical activity depend on the type of exercise. The two types of exercise (isotonic and isometric exercise) involve the use of large muscle masses that increase venous return and left ventricular end-diastolic volume. Along with stimulating the sympathetic adrenergic system, said masses increase heart rate, blood pressure, cardiac output and myocardial contractility. The aim of these responses is to increase the oxygen supply to the myocardium with an increased blood flow, which in the context of these anomalies is compromised.6,9,10
These anomalies are more common in individuals with congenital heart disease (particularly tetralogy of Fallot, transposition of the great arteries and some forms of pulmonary atresia) than in individuals with a structurally normal heart. Symptoms usually occur earlier, as does the diagnosis. This affects the treatment and prognosis of the underlying disease.7,11
The real incidence of CCAAs in the general population remains unclear, ranging from 0.2% to 5.6%. This variation depends on the method (autopsy vs. angiography), the diagnostic criteria and the population studied.4,12,13 There are no known differences in incidence between ethnicities and genders.2
CCAAs are often only detected in autopsy. Their diagnosis is a challenge that requires a high level of clinical suspicion, since most individuals are asymptomatic and have a normal physical examination. Symptoms, such as chest pain, dyspnea or syncope, occur in only 18% to 30% of patients.4,14,15
This study aims to highlight the main primary CCAAs with hemodynamic significance, particularly anomalous origin of coronary arteries from the opposite sinus and anomalous left coronary artery from the pulmonary artery.
Anatomy and embryology of the coronary arteriesA proper study of CCAAs involves briefly reviewing coronary artery development and anatomy.
Determining what is normal in the anatomy of coronary arteries is a challenge. Angelini et al. classified any anatomy present in more than 1% of the general population as normal, including a wide range of anatomical variants. Thus, by definition, CCAAs occur in less than 1% of the population.4,5,16
Usually, the left coronary artery arises from the left coronary sinus, taking a course to the left and posterior to the trunk of the pulmonary artery, and dividing into two arteries: the left anterior descending artery and the circumflex artery. The former is located in the anterior interventricular sulcus, which is responsible for blood supply to the anterior two thirds of the basal region of the septum and its middle and apical region. The latter is located in the left coronary sulcus and supplies the wall of the left ventricle.2,17
On the other side, the right coronary artery arises from the right coronary sinus and is responsible for blood supply to the right ventricular wall. It follows a course anterior and to the right, between the right atrium and the pulmonary artery, located in the coronary sulcus. Along this course, it gives rise to several branches.2,17,18 Regarding variants of normal, the posterior descending artery supplies the inferior wall and the inferior third of the septum. Said artery may originate in the right coronary artery (right dominance, the most common), the left coronary artery (left dominance) or both (codominance).16 There are no data in the literature from which it may be inferred that dominance affects the survival of individuals with CCAAs. However, it is known that in a left-dominant system, the right coronary artery does not contribute to left ventricular perfusion. This may have consequences during a cardiac event.
During the initial phases of human fetal development, vascular sinusoids develop within the embryonic myocardium. As the myocardium becomes more compact, the sinusoids disappear and a network of veins, arteries and capillaries develops. These vessels connect with other mediastinal vessels at approximately 32 days of gestation. The primitive coronary vessels appear around the seventh week of gestation, after the formation of the aorta from the division of the truncus arteriosus. As the coronary artery network develops, endothelial buds appear at the base of the truncus arteriosus. These later join with the coronary artery network, which develops from the sinusoids, establishing the definitive coronary artery circulation. Abnormal involution, bud position or septation of the truncus arteriosus may lead to the development of an abnormal origin of the coronary arteries.19,20
Given this complex embryology, it is expected that deviations in developmental may result in congenital anomalies.19
ClassificationClassification of CCAAs is a controversial topic and several models have been proposed.
The first is from 1969 and was proposed by Ogden.21 It divides them into three categories: minor anomalies, major anomalies and secondary anomalies. However, this system does not emphasize the clinical importance of certain abnormalities.5,22
The classification initially proposed by Angelini23 in 1989 was subsequently updated. It is currently one of the most used and divides CCAAs into: a) anomalies of origination and course; b) anomalies of intrinsic coronary arterial anatomy; c) anomalies of coronary termination; and d) anomalous anastomotic vessels. The basic principle of this system is that the name of an artery is determined by the territory it supplies blood to, and not based on its origin or initial course.2,8 According to Angelini, the following characteristics are considered normal: the presence of two to four ostia, in the right and left coronary sinuses; a proximal orientation of 45° to 90° from the aortic wall; the presence of a common trunk, located on the left, giving rise to the left anterior descending artery and circumflex artery; a middle subepicardial segment, with branches appropriate for the dependent myocardium; and termination of the entire system in a capillary network.2,5,8
Although the above classification is detailed, comprehensive, and understandable, in 2000, Dodge-Khatami et al. introduced a new system of nomenclature and classification as an integral part of the International Congenital Heart Surgery Nomenclature and Database Project of the European Association for Cardio-Thoracic Surgery and the Society of Thoracic Surgeons. This system was adopted to classify diagnoses and procedures in the Congenital Heart Surgery Database, based on various levels of hierarchy. This classification has been used to record anomalous aortic origin of a coronary artery in order to develop guidelines for its treatment and monitoring.5,12
In 2003, a new classification emerged, proposed by Rigatelli et al., with the aim of being more practical and simpler. It standardized the diagnostic criteria and the placed CCAA groups into seven categories: hypoplasia/atresia, a single coronary artery (hyperdominance), fistula, originating from other arteries, originating from the wrong coronary sinus, splitting (separate origins from the left anterior descending artery and circumflex artery) and tunneling.24 This classification is more simplistic, whereas the Angelini classification establishes the relationship between clinical situation and anomaly, by referring to the territory supplied.
Anomalous aortic origin of a coronary artery (AAOCA)AAOCA is a congenital anomaly of the origin or course of a coronary artery originating in the aorta.25 The incidence of this anomaly ranges from 0.28% to 1.74% in studies conducted with coronary angiography.26 This group includes anomalous origin of coronary arteries from the opposite sinus, which is one of the main causes of sudden death.25
The prevalence of AAOCA with the origin of the right coronary artery in the left coronary sinus ranges from 0.06% to 0.9%. The prevalence is lower (approximately 0.025% to 0.15%) for the origin of the left coronary artery in the right coronary sinus.1,25
In 1962, an anomalous left coronary artery with its origin in the right coronary sinus was described in a healthy boy found dead after a cross-country race. Later, in 1974, Cheitlin et al. demonstrated that several autopsied patients with anomalous origin of coronary arteries from the opposite sinus had experienced sudden death.1
The annual incidence of sudden cardiac death among young athletes with AAOCA arising from the opposite sinus is between 1:43800 and 1:200000, corresponding to 14–17% of all cardiac deaths. Most deaths associated with this abnormality occur unexpectedly in healthy children or young athletes during or immediately after physical exercise, and are more commonly associated with sports such as basketball, soccer, swimming, and track and field.25,27
Most of these events occur between the ages of 10 and 30.25 Sudden cardiac death in older individuals is less common, and is normally associated with atherosclerotic events. This lower risk is likely explained by the lower rate of participation in high-intensity sports.13
Due to the age distribution of its incidence, this cause of death has a major social impact as a result of the high number of years of life potentially lost.28
Although anomalous origin of the right coronary artery in the left coronary sinus is approximately six times more prevalent than anomalous origin of the left coronary artery, the latter seems to be responsible for up to 85% of cases of sudden cardiac death related to AAOCA, which makes it more fatal.13,25 Occlusion of the left coronary artery results in a massive anterolateral and septal ischemia, followed by tachycardia/ventricular fibrillation or extensive myocardial infarction and cardiogenic shock. If the occlusion is in the proximal region of the right coronary artery, the resulting infarction occurs in the inferior region of the myocardium and is usually not fatal.6
Five coronary artery trajectories originating in contralateral coronary sinuses are possible: (1) pre-pulmonary, which usually has no hemodynamic consequences, frequently involves the left coronary artery and is particularly common in the tetralogy of Fallot; (2) retroaortic, with a course posterior to the aortic root, a variant which also has no hemodynamic significance and frequently involves the right coronary artery; (3) interarterial, located between the aorta and the pulmonary artery and associated with a poorer prognosis; (4) intraseptal, with subpulmonary course, in which the most involved artery is the left coronary artery; and (5) retrocardiac, in the posterior atrioventricular sulcus.16,29
Brothers et al.14 presented the first case series of families with AAOCA with an interarterial course in the pediatric population. This led to questioning whether or not a genetic link comes into play. In fact, there are several genetic causes of sudden death in children, so screening is recommended in other family members. No genetic abnormalities responsible for CCAAs have been identified to date. However, due to the demonstrated possibility of family association, echocardiography screening of direct family members may help prevent sudden death.14
Despite the clear association between AAOCA and sudden death, no studies have demonstrated the mechanism that explains this association. It is believed that the coronary artery undergoes compression/occlusion during physical exercise, leading to myocardial ischemia and tachycardia or ventricular fibrillation.13,25 The mechanism responsible for this compression/occlusion is not yet clear, but several hypotheses have been proposed, such as those below.
Interarterial courseCoronary arteries with their origin in the opposite sinus may have a trajectory between the aorta and the pulmonary artery. This can lead to the anomalous artery being compressed between these two great vessels during vigorous exercise. However, studies with intravascular ultrasound have shown that the narrowing of the proximal segment of the anomalous coronary artery occurs even when the pulmonary artery distances itself during the cardiac cycle.25 Moreover, the low pressure found in the pulmonary artery compared to the pressure of the coronary artery, even during physical exertion, also does not seem to explain this compression.13,25
Intramural courseMany patients with AAOCA have a variable length of the proximal segment of the coronary artery, with a trajectory within the aortic wall before entry into the mediastinum.25 Thus, it is plausible that the expansion of the aorta during physical exercise may compress this intramural portion of the coronary artery.13,25 This segmental length is a measurement of severity.2
Several studies by Angelini2 have demonstrated the importance of this abnormality, describing several mechanisms of stenosis. Intravascular ultrasound found that the circumference of the intramural segment of the artery, with ectopic origin, is 20–70% less than the extramural distal segment of the same coronary artery.2,25 This discrepancy was quantified in a hypoplasia index. Arteries that congenitally have this course in the aortic wall probably do not grow normally before or after birth.2 The cross-section of the intramural segment is not circular, but ovoid. Lateral compression of an artery with these characteristics results in a smaller area than an artery with a circular shape and the same circumference.2,25 This abnormality can be assessed using the asymmetry ratio. Furthermore, Angelini2 simulated vigorous physical exercise with a dobutamine stress test or fluid overload. He demonstrated that the smaller diameter segment becomes gradually more compressed during systole and that this behavior is related to changes in systolic volume (and consequently in the pulsatility of the ascending aorta) and to tachycardia.2,25
With age, it is likely that the thickening and increased stiffness of the aortic wall reduce aortic distensibility, thereby reducing the risk of sudden death in the older population.6
Ostial anomaliesDue to the oblique course of the anomalous coronary artery in the proximal region, the coronary ostium may be a slit rather than circular. Thus, it may collapse, exerting a valve effect. This may occur due to aortic expansion during systole and during exercise, compromising blood supply. In addition, coronary ostial stenosis is common. These abnormalities can restrict the increased blood flow inherent in physical exercise.6,13,25
Acute angle takeoffWhile the coronary artery is usually perpendicular in an individual with normal anatomy, an anomalous coronary artery may start at an acute angle from the aorta, which may alter blood flow.13,26 This oblique origin can result in intimal lesion and coronary spasm, triggered by sympathetic adrenergic stimulation and resulting from physical activity.6 This anomaly can make the artery more susceptible to atherosclerosis due to alterations in flow.16
The clinical presentation of patients with AAOCAs varies greatly. It can manifest as nonspecific chest pain, palpitations, dyspnea or syncope. These symptoms are most often related with physical exertion. However, individuals may be asymptomatic, with the first presentation being sudden death.1,25,30 One study showed that around 50% of patients who had this anomaly and died suddenly after physical exercise were asymptomatic.13
The physical exam is most often normal, unless there is an associated structural cardiac injury. As such, diagnosing these patients is challenging.25 Most AAOCAs are discovered by chance, during coronary angiography performed in patients with ischemic or valve disease.26
An electrocardiogram (ECG) or stress test may reveal alterations suggestive of ischemia (Q waves or ST segment alterations) or cardiac arrhythmia, but in most cases they are normal. These alterations can lead to a suspicion of anomalies, especially in children and young adults. Moreover, they can be the starting point for conducting other complementary diagnostic tests.29,31
Imaging tests play an important role in detecting, categorizing and stratifying risk in patients with AAOCAs.15
The role of myocardial perfusion imaging is not fully clear. Several studies have shown that only a small proportion of individuals (who underwent surgery) had alterations on this test.25
Transthoracic echocardiography is the most commonly used imaging method for this diagnosis. It is an excellent method that is widely available, inexpensive, non-invasive, and that does not expose individuals to ionizing radiation. For these reasons it should be the method of choice. It can identify anomalous coronary arteries in young individuals, since the origin and proximal segments of the coronary arteries can be very easily viewed. Identifying the origin of the coronary arteries should be a routine part of any echocardiographic exam.6,15 However, several studies have shown variable sensitivity. This is explained by the method's dependence on the operator, the patient's age and the anomaly in question.31,32 Transesophageal echocardiography can also reveal the origin of both coronary arteries.6,26
Conventional coronary angiography was once considered the gold standard in diagnosing CCAAs. However, it is an invasive method that uses ionizing radiation. With the advances made in imaging techniques, especially with magnetic resonance and computed tomography, three-dimensional assessment of the origin and course of the coronary arteries has become possible.31,33
Computed tomography angiography (CTA) or magnetic resonance angiography (MRA) are non-invasive methods that are often used to confirm the diagnosis of these anomalies.25
MRA can provide information about myocardial perfusion and function, viability, and arterial flow without the use of ionizing radiation. However, sedation is often necessary in children. More importantly, MRA does not have the necessary spatial resolution or suitable contrast to analyze the ostium and the proximal course of the coronary artery and thus cannot distinguish an intramural course from an interarterial one. It also does not provide information about dynamic changes in the coronary lumen during the cardiac cycle.25,34
Recent advances in cardiac CTA have allowed more information to be collected with this technique, while exposing individuals to lower doses of ionizing radiation. With CTA, the location, shape and angle of the origin of each coronary artery, its course and its relationship to the great vessels can be precisely determined. Several studies have reported using this auxiliary method to determine the coronary artery anatomy, as evidenced by Amado et al.32 in a retrospective study that proved the usefulness of this test for detecting CCAAs.9,34,35
A diagnostic protocol by Barriales Villa26 for patients under 35 years of age suspected to have these anomalies states that, in the initial approach, a transthoracic echocardiography should be performed. This enables identification of the ostium, but has limitations in identifying the course of the coronary arteries. If doubt persists or if the origin and course of the coronary arteries cannot be viewed, a transesophageal echocardiography or CTA should be performed.26
The 36th Bethesda Conference, which led to a publication in April 2005, recommends that coronary artery anomalies should be considered in athletes with exertional syncope or symptomatic ventricular arrhythmias, and investigated using auxiliary studies, such as echocardiography, cardiac magnetic resonance or computed tomography. Coronary angiography is indicated if previous studies have failed to provide a diagnosis.36
Therapeutic approach is one of the most controversial issues, since there are no official guidelines.26,27 The European Society of Cardiology guidelines on congenital heart disease do not include the therapeutic approach in these patients.37
There is consensus that surgery should be offered as an option in individuals under 30 years of age with evidence of ischemia or ventricular arrhythmias and with more severe symptoms, such as syncope. However, the management of other patients is still controversial, particularly as regards asymptomatic individuals under 30 years of age with no documented ischemia, and symptomatic patients with no documented ischemia, in whom the symptoms do not reliably indicate ischemia.1,6 It is important to note that sudden death is a rare event in individuals over 30–35 years of age. In older asymptomatic individuals, the risk of complications from surgery exceeds the therapeutic benefit, so surgery should be avoided.1,27
With regard to physical exercise, recommendations suggest restricting the practice of “competitive sports”. This is vague and compliance by children and young adults can be problematic. In addition, the possibility of sudden death is not avoided with minimal exercise. On the other hand, in active children and young adults, the benefits may outweigh the risks. This is because regular physical activity is an important component of growth and development, with well-known benefits for psychological and physical health. Several authors suggest that physical education curricular activities can be performed as long as they do not involve strenuous exercise.1,25
Vigilance or medical treatment with beta blockers have been proposed, with apparent success. However, the studies that involve this approach have follow-up periods of less than two years. There is only one study38 with a five-year follow-up, in which beta blockers were used in individuals with a mean age of 55 years and with anomalous origin of the right coronary artery from the opposite sinus. These individuals had no atherosclerosis and did not undergo surgery, and no cases of sudden death were reported. Additionally, these drugs are used in other conditions which predispose patients to sudden death, such as hypertrophic cardiomyopathy or congenital long QT syndrome. Therefore, the decision to initiate prophylactic therapy with beta blockers should be carefully assessed.1,39
Surgical treatment includes various techniques: coronary bypass, reimplantation of the anomalous vessel in the correct coronary sinus, ostioplasty and translocation of the pulmonary artery to reduce the risk of compression of the anomalous vessel (if it has an interarterial course). Recently, unroofing of the anomalous vessel in its intramural segment has become one of the most commonly used options.4,25 However, this technique risks affecting the aortic valve and leading to insufficiency.1
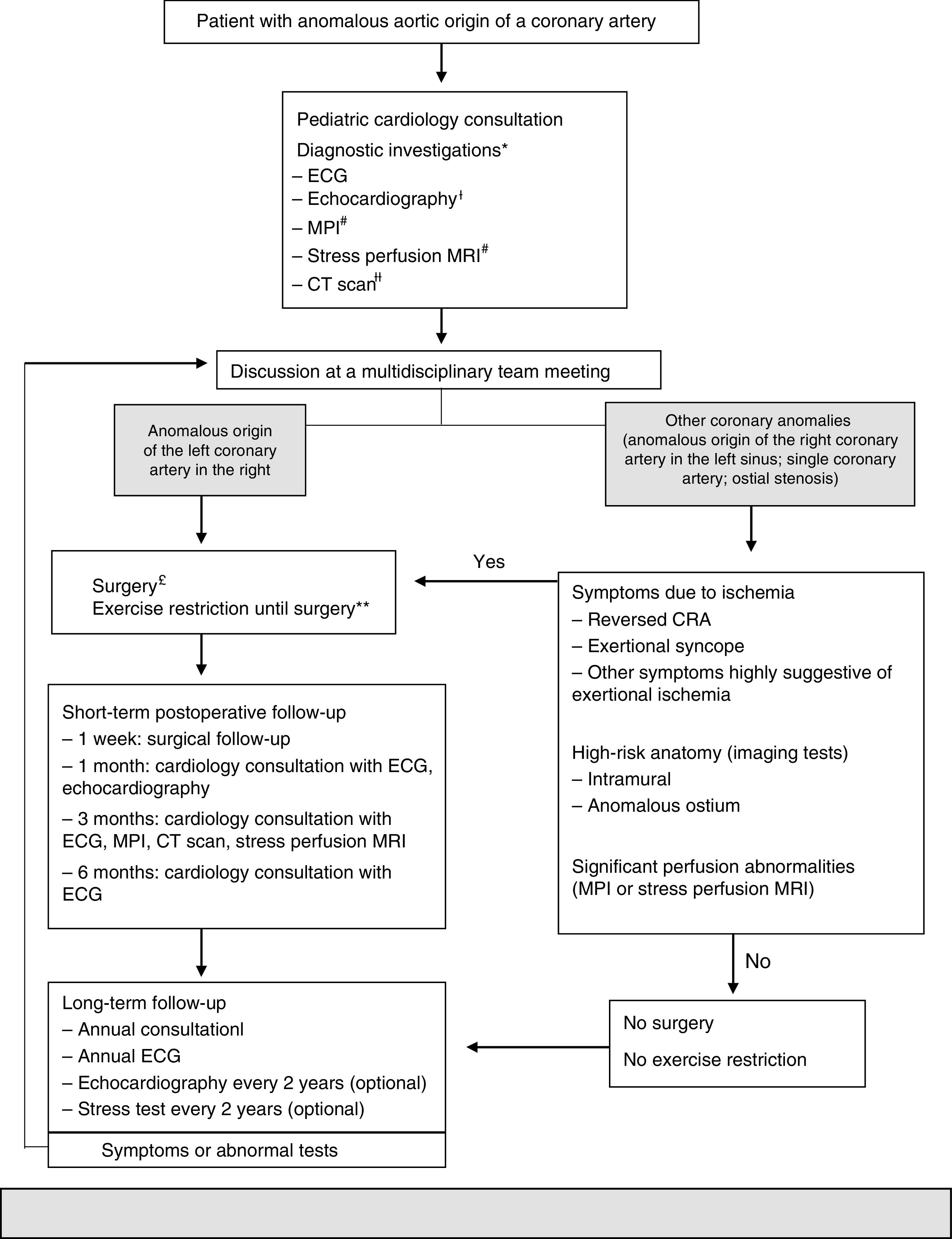
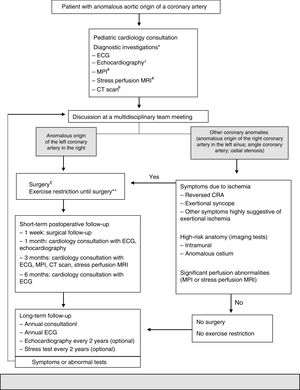
Due to the controversies surrounding this topic, Mery et al.25 created a clinical algorithm (Figure 1) based on the evidence in the literature to date. It is an attempt to provide initial guidance regarding these patients and can be reviewed according to the evidence.25
In 2012, Mery et al. created a multidisciplinary program to generate a clinical algorithm.
*Additional tests may be ordered, depending on the clinical assessment.
¿An echocardiogram performed externally does not need to be repeated if the test was adequate.
#These tests are not necessary in patients with reversed cardiorespiratory arrest. MR depends on the patient's age and cooperation.
¿¿A CT performed externally can be used if the images are available and the test provides all the information necessary to make a decision.
£Unroofing for a significant intramural segment; creation of a new ostium or translocation if the intramural segment is behind the commissure; translocation of the coronary artery or ostioplasty if there is no intramural segment. In patients 10–35 years of age; others on a case-by-case basis.
**Restricted participation in competitive sports or exercise with a moderate or high dynamic component (>40% use of maximum oxygen, e.g. swimming, soccer, basketball).
++Postoperative individuals may do any exercise or practice competitive sports depending on the month-3 assessment, which includes results of the MPI, stress perfusion MRI or CT scan.
AAOCA: anomalous aortic origin of a coronary artery; CRA: cardiorespiratory arrest; CT: computed tomography; ECG: electrocardiogram; MPI: myocardial perfusion imaging; MRI: magnetic resonance imaging.
Adapted from 2015 Texas Children's Hospital. Copyright© 2015 Texas Children's Hospital. All rights reserved.
This algorithm recommends that all patients with AAOCA be monitored by two cardiologists and undergo a set of standardized tests: echocardiography, CTA and myocardial perfusion imaging. Subsequently, all patients are discussed in a multidisciplinary program dedicated to CCAAs in order to decide on the best strategy to follow. Surgery is proposed for individuals whose left coronary arteries arise from the opposite sinus. For individuals with other anomalies, surgery is proposed if they are symptomatic or if diagnostic investigations reveal high-risk abnormalities, such as a long intramural course of the anomalous coronary artery or dynamic changes in the ostium during the cardiac cycle. All patients are clinically monitored at specific intervals. Restricting dynamic physical exercise is recommended only to patients who are pending or have decided against surgery. For patients who undergo surgery, physical activity is restricted for three months after the procedure. The restriction is lifted after that time if the patient is asymptomatic and if investigations do not show any abnormalities.
Finally, it is important to emphasize that any explanation for sudden death related to AAOCAs should take into account certain facts, which have been well documented: (a) sudden death is frequently associated with or immediately follows vigorous physical exercise; (b) most of the time, individuals who die suddenly perform the same or a higher level of physical activity without any symptoms, which suggests that this mechanism of obstruction/compression is intermittent and unpredictable; (c) sudden death associated with these anomalies is rare after 30 years of age; (d) ECG, stress test and echocardiography before or after an episode of reversed sudden cardiorespiratory arrest are usually negative; as such, it is likely that myocardial ischemia will not be induced in these patients by reproducing the exercise that precipitated the event; (e) regardless of the mechanism responsible for occlusion/compression of the anomalous artery, it is likely to involve the most proximal portion of the artery. In patients who survive myocardial ischemia, the territory supplied by this artery becomes a substrate for malignant ventricular arrhythmias.6
Anomalous left coronary artery from the pulmonary arteryAlthough this congenital anomaly is rarer than AAOCA, it is of great clinical significance. This anomaly is also named Bland-White-Garland syndrome after the authors who first clinically described it in 193340 following the autopsy of a 3-month-old infant. The child had difficulty feeding, cardiomegaly and evidence of left ventricular lesion on ECG. However, this anomaly was first described anatomically by Brooks41 in 1883. The vast majority die in the first year of life if surgery is not performed, which is the case for up to 90% of affected children. Thus, it is uncommon for individuals with this abnormality to reach adulthood without undergoing surgery.42,43 ALCAPA has an incidence of 1:300000 live births. This anomaly is an important differential diagnosis in children with heart failure and may be associated with other malformations, such as atrial septal defect, ventricular septal defect or coarctation of the aorta.43,44
Several theories have been described to explain the origin of a coronary artery in the pulmonary artery, specifically related to the embryological division of the truncus arteriosus. Assuming that the coronary arteries emerge as two endothelial buds, there may be displacement of the site of origin of one or both coronary arteries to the region of the truncus arteriosus that would give rise to the pulmonary artery. Another possibility involves the anomalous division of the truncus by the developing septum, and which would result in the incorporation of one of the origins of the coronary arteries into the pulmonary artery.42
Patients with ALCAPA generally have early symptoms, usually in the first year of life, with chronic ischemia and consequent heart failure. Infants typically have feeding problems, irritability, diaphoresis, tachypnea, tachycardia, and sometimes chest pain due to myocardial ischemia. Patients may be asymptomatic during infancy if there is sufficient collateral circulation between the two coronary arteries. In a later phase, dyspnea, chest pain, syncope, intolerance to physical exercises or, finally, sudden death may occur due to acute ischemia during exercise or ventricular arrhythmias produced from myocardial scar tissue.42
Four phases have been proposed to explain the symptoms and progression of this anomaly.43 In the first month of life, physiological pulmonary hypertension and fetal hemoglobin provide perfusion and oxygenation to the myocardium, so individuals are asymptomatic.16,43 Then, in a second phase, a reduction in pulmonary pressure and oxygen levels16,43 often causes decompensation, leading to decreased coronary perfusion and ischemia of the anterolateral wall. This ischemia is aggravated during feeding or crying, situations with greater oxygen demands. Chronic ischemia with consequent heart failure and associated mitral insufficiency may affect diagnosis, with the occurrence of QS waves in precordial leads, left ventricular dilation, and functional mitral insufficiency in the most severe cases. If this phase is overcome, then compensatory changes and remodeling of the myocardium occur over time.43 The third phase is considered to be survival until adulthood, where intercoronary collateral vessels develop from the right-side circulation and maintain the perfusion of the left coronary artery territory.16,43 In this phase, there is a shunt from the right coronary artery, via collateral vessels, to the left coronary artery.43 Pressure becomes greater here than in the pulmonary artery, inverting the flow of the anomalous left coronary artery to the pulmonary artery.16 This is an example of coronary steal. Patients become symptomatic and may experience angina, fatigue, dyspnea, palpitations, ventricular arrhythmias, pulmonary hypertension and sudden death.16,43 As they age, these individuals adapt not only to the chronic ischemia caused by the anomalous left coronary artery, but also to the shunt between the arterial and venous systems. In addition to the formation of collateral vessels and right coronary artery dominance, they develop ostial stenosis or restriction of the opening of the anomalous left coronary artery into the pulmonary artery, reducing the shunt with a consequent reduction in coronary steal. Finally, collaterals from the bronchial arteries to the myocardium develop, increasing perfusion. Alterations in the development of these adaptations contribute to morbidity and mortality, since systolic dysfunction and left ventricular dilation result from chronic myocardial ischemia.45,46
The ECGs of patients with this anomaly may show evidence of ischemia or infarction of the anterolateral wall (alterations in the ST segment, Q waves in I, aVL, V5 and V6). However, 20–45% of patients do not have these abnormalities.29
Diagnosis of this anomaly requires a high degree of clinical suspicion, which in the past was confirmed by coronary angiography.42,43 This is based not only on the observation of the origin of the left coronary artery in the pulmonary artery, but also on the retrograde flow from the anomalous left coronary artery to the pulmonary artery. Currently, transthoracic echocardiography enables observing this highly suggestive characteristic, thereby confirming the diagnosis. It also enables viewing the origin of the artery, right coronary artery dilation, and cardiac dilation with associated heart failure and mitral insufficiency.31,42,43 Magnetic resonance and computed tomography are also used to confirm diagnosis, although they are particularly important in postoperative follow-up.29
The diagnosis of this congenital anomaly in a seriously ill child is an indication for urgent surgery. In children, the aim is to reestablish coronary perfusion, preserving the myocardium and enabling recovery of left ventricular function. The standard definitive treatment for ALCAPA is direct reimplantation of the coronary artery in the aorta, thereby reestablishing the normal coronary system.31,46,47 When this technique is not possible, alternative techniques can be used, such as the creation of an intrapulmonary tunnel, connecting the ostium of the anomalous coronary artery to the aorta (Takeuchi technique).47
Anomalies in the origin of the right coronary artery from the pulmonary artery are extremely rare, with an incidence of 0.002%. Approximately 25–30% are associated with other congenital cardiac anomalies.45 These abnormalities are asymptomatic in more than 75% of cases, with no evidence of myocardial ischemia. Few cases of sudden cardiac death and heart failure have been reported.11,29,48
ConclusionCCAAs are a heterogeneous group of rare congenital abnormalities, the manifestations of which vary greatly. Although most are benign, some are potentially serious and cause myocardial ischemia and sudden death. Most of these anomalies are asymptomatic and physical examinations find no abnormalities. As such, they require a high degree of clinical suspicion.
Anomalous origin of coronary arteries from the opposite sinus is among the most important of the CCAAs. It is most often associated with myocardial ischemia and sudden death in particular. Clinical suspicion should be high, especially in young individuals with exertional chest pain, dyspnea, or syncope. How to treat this type of anomaly is one of the most controversial issues, as there are no official clinical guidelines in this regard. This should be the object of further investigation.
The nature and age of presentation of ALCAPA vary and are dependent on existing collateral circulation. This anomaly should be suspected in children with symptoms of heart failure, ECG abnormalities and decreased left ventricular systolic function. Surgical treatment has good results even in patients with severe left ventricular dysfunction and mitral insufficiency.
Due to the hemodynamic complications that these anomalies cause, early diagnosis and treatment are crucial.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Silva A, Baptista MJ, Araújo E. Anomalias congénitas das artérias coronárias. Rev Port Cardiol. 2018;37:341–350.


 40% use of maximum oxygen, e.g. swimming, soccer, basketball). ++Postoperative individuals may do any exercise or practice competitive sports depending on the month-3 assessment, which includes results of the MPI, stress perfusion MRI or CT scan. AAOCA: anomalous aortic origin of a coronary artery; CRA: cardiorespiratory arrest; CT: computed tomography; ECG: electrocardiogram; MPI: myocardial perfusion imaging; MRI: magnetic resonance imaging. Adapted from 2015 Texas Children's Hospital. Copyright© 2015 Texas Children's Hospital. All rights reserved.' title='In 2012, Mery et al. created a multidisciplinary program to generate a clinical algorithm. *Additional tests may be ordered, depending on the clinical assessment. ¿An echocardiogram performed externally does not need to be repeated if the test was adequate. #These tests are not necessary in patients with reversed cardiorespiratory arrest. MR depends on the patient's age and cooperation. ¿¿A CT performed externally can be used if the images are available and the test provides all the information necessary to make a decision. £Unroofing for a significant intramural segment; creation of a new ostium or translocation if the intramural segment is behind the commissure; translocation of the coronary artery or ostioplasty if there is no intramural segment. In patients 10–35 years of age; others on a case-by-case basis. **Restricted participation in competitive sports or exercise with a moderate or high dynamic component (>40% use of maximum oxygen, e.g. swimming, soccer, basketball). ++Postoperative individuals may do any exercise or practice competitive sports depending on the month-3 assessment, which includes results of the MPI, stress perfusion MRI or CT scan. AAOCA: anomalous aortic origin of a coronary artery; CRA: cardiorespiratory arrest; CT: computed tomography; ECG: electrocardiogram; MPI: myocardial perfusion imaging; MRI: magnetic resonance imaging. Adapted from 2015 Texas Children's Hospital. Copyright© 2015 Texas Children's Hospital. All rights reserved.'/>
40% use of maximum oxygen, e.g. swimming, soccer, basketball). ++Postoperative individuals may do any exercise or practice competitive sports depending on the month-3 assessment, which includes results of the MPI, stress perfusion MRI or CT scan. AAOCA: anomalous aortic origin of a coronary artery; CRA: cardiorespiratory arrest; CT: computed tomography; ECG: electrocardiogram; MPI: myocardial perfusion imaging; MRI: magnetic resonance imaging. Adapted from 2015 Texas Children's Hospital. Copyright© 2015 Texas Children's Hospital. All rights reserved.' title='In 2012, Mery et al. created a multidisciplinary program to generate a clinical algorithm. *Additional tests may be ordered, depending on the clinical assessment. ¿An echocardiogram performed externally does not need to be repeated if the test was adequate. #These tests are not necessary in patients with reversed cardiorespiratory arrest. MR depends on the patient's age and cooperation. ¿¿A CT performed externally can be used if the images are available and the test provides all the information necessary to make a decision. £Unroofing for a significant intramural segment; creation of a new ostium or translocation if the intramural segment is behind the commissure; translocation of the coronary artery or ostioplasty if there is no intramural segment. In patients 10–35 years of age; others on a case-by-case basis. **Restricted participation in competitive sports or exercise with a moderate or high dynamic component (>40% use of maximum oxygen, e.g. swimming, soccer, basketball). ++Postoperative individuals may do any exercise or practice competitive sports depending on the month-3 assessment, which includes results of the MPI, stress perfusion MRI or CT scan. AAOCA: anomalous aortic origin of a coronary artery; CRA: cardiorespiratory arrest; CT: computed tomography; ECG: electrocardiogram; MPI: myocardial perfusion imaging; MRI: magnetic resonance imaging. Adapted from 2015 Texas Children's Hospital. Copyright© 2015 Texas Children's Hospital. All rights reserved.'/>

