Pericardial effusion is a common complication in clinical situations such as cardiothoracic surgery and cancer, in which pericardiocentesis may be essential. Pericardiocentesis can be guided by different imaging techniques, most commonly echocardiography. Computed tomography (CT) has significant advantages but there is still little evidence supporting its use in this context. In this work we describe our experience with CT-guided pericardiocentesis (CTP) in a single center.
MethodsPatients referred for CTP between August 2008 and February 2014 were retrospectively analyzed. We assessed demographics, etiology of the effusion, international normalized ratio during the procedure, radiation doses, success rate and complications. Results were compared with those in the literature.
ResultsDuring this period, 51 procedures were performed, in 46 patients. Five patients underwent a repeat procedure due to recurrence of effusion. The most common etiologies were post-surgical (48%, 22 patients) and neoplasm-related (17%, eight patients).
Drainage was considered completely successful in 46 cases (90%), partially successful in two (4%) and unsuccessful in three (6%).
The median duration of the procedure was 65 min (interquartile range 50–80) and median effective radiation exposure was 3.3 mSv (interquartile range 2.4–5.2 mSv). There were no significant adverse events related to the procedure.
ConclusionsBy providing high-definition three-dimensional images, CTP enables accurate positioning of pericardiocentesis material. It was shown to be an accurate, effective and safe method, in agreement with previous findings. CTP should be considered a good option in centers with CT facilities.
O derrame pericárdico é uma complicação temível em várias situações clínicas, em que a pericardiocentese pode ter um papel fundamental. Esta pode ser guiada por vários métodos de imagem, sobretudo ecocardiografia. A tomografia computorizada (TC) tem várias vantagens, mas o seu uso tem ainda fraca evidência clínica. Neste trabalho é reportada a experiência de um centro em pericardiocentese guiada por TC (P-TC).
MétodosForam analisados retrospetivamente pacientes (pts) referenciados para P-TC durante o período de agosto de 2008 a fevereiro de 2014. Foi avaliada a demografia, etiologia do derrame, INR, radiação, sucesso e complicações, sendo os resultados comparados aos publicados na literatura.
ResultadosDurante este período, foram realizados 51 procedimentos em 46 doentes. Cinco doentes repetiram o procedimento devido a recorrência do derrame. A idade média foi de 63±13,8 anos. As etiologias mais frequentes foram pós-cirúrgica (48%, 22 pts) e neoplasia (17%, oito pts).
A drenagem foi considerada completa em 90% (46) dos casos, parcial em 4% (dois) e ineficaz em 6% (três).
O procedimento teve uma duração mediana de 65 minutos (Q1-Q3 50-80 minutos) e a exposição de radiação foi de 3,3 mSv (Q1-Q3 2,4-5,2 mSv). Não foram detetadas complicações imediatas relevantes.
ConclusõesAo providenciar imagens de alta definição em três dimensões, a P-TC permite o posicionamento preciso do material de pericardiocentese, tendo demonstrado nesta série ser um método eficaz e seguro, indo ao encontro da informação previamente publicada. A P-TC deve, por isso, ser considerada uma boa opção em centros com disponibilidade de TC.
There are many clinical scenarios in which pericardial effusion may develop.1 It can be caused by bleeding into the pericardial space or other fluid accumulation. Etiology may be inflammatory/infectious, traumatic, post-operative,2 or related to cancer or other chronic conditions such as uremia.3 Its main adverse consequence is its potential impact on cardiac hemodynamics, which will depend mainly on the volume and rate of development of the effusion.4 Clinical presentation of a significant pericardial effusion usually includes lightheadedness, chest discomfort, dyspnea, anxiety, tachycardia and hypotension.5 Echocardiography is the imaging modality of choice for diagnosis and for estimating hemodynamic impairment, through assessment of right heart chamber dynamics. Other diagnostic tools may be required for etiologic investigation or anatomical definition, such as computed tomography (CT) and cardiovascular magnetic resonance.6,7 Pericardiocentesis is usually indicated when hemodynamic impact is significant or when needed for etiologic diagnosis.8–10
As it is an invasive procedure, there is always a significant risk of complications, which may be reduced with enhanced control in needle positioning, in order to avoid unintended puncture of structures. Electrocardiogram-guided11 and fluoroscopy-guided12 techniques have been described. Guidance in recent years has been mainly through echocardiography13–16 (offline or real-time) because of its improved safety over a blind approach. CT-guided pericardiocentesis (CTP) is also an option in centers with access to and expertise in this technique,17,18 with the advantage of detailed three-dimensional imaging, which enables fine needle positioning.
This work reports on our experience with CTP.
MethodsAll procedures were performed by experienced cardiologists or cardiac surgeons, using a Seldinger technique according to previously described protocols.17 CT guidance was obtained using a 64-slice scanner (Siemens SOMATOM Sensation, Erlangen, Germany) (Figure 1). Patients provided informed consent. During the procedure they were asked to breathe steadily and abstain from moving or breathing deeply. Local anesthesia with subcutaneous lidocaine was performed in every patient and light sedation (diazepam 5 mg orally) was administered when needed.
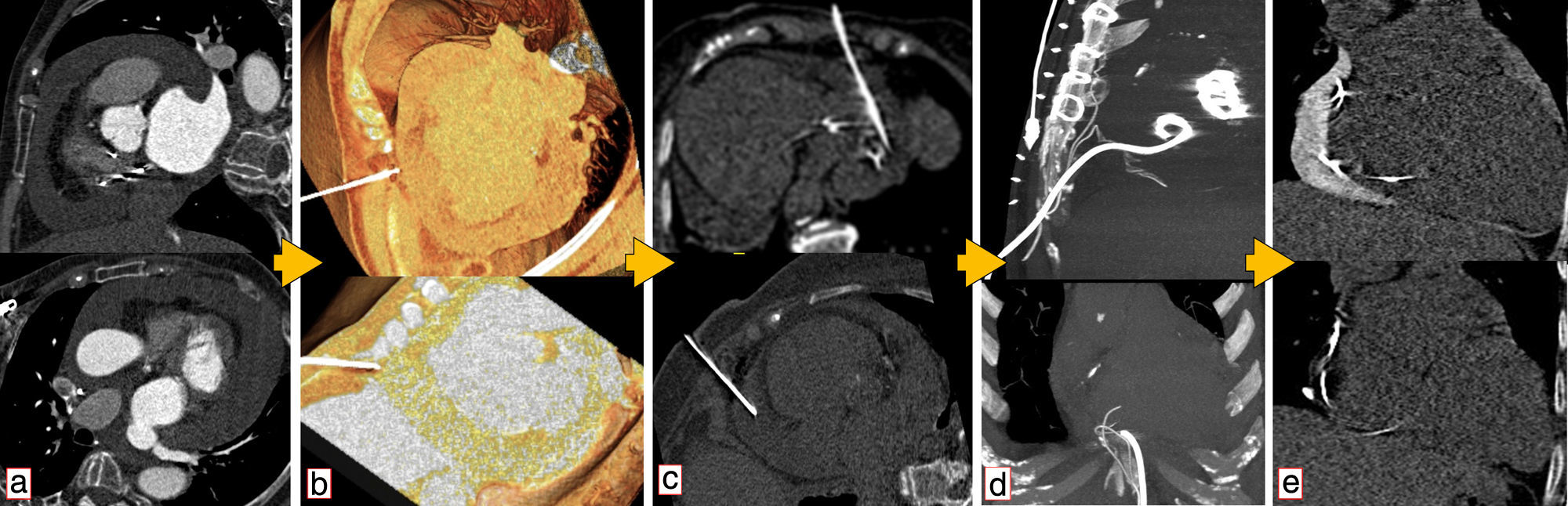
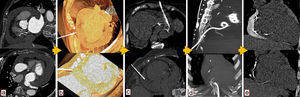
Use of computed tomography in pericardiocentesis. (a) Characterization and assessment of effusion; (b) determination of best entry point and needle orientation; (c) control of needle progression and relation with surrounding structures; (d) pigtail positioning; (e) final result and control of evolution.
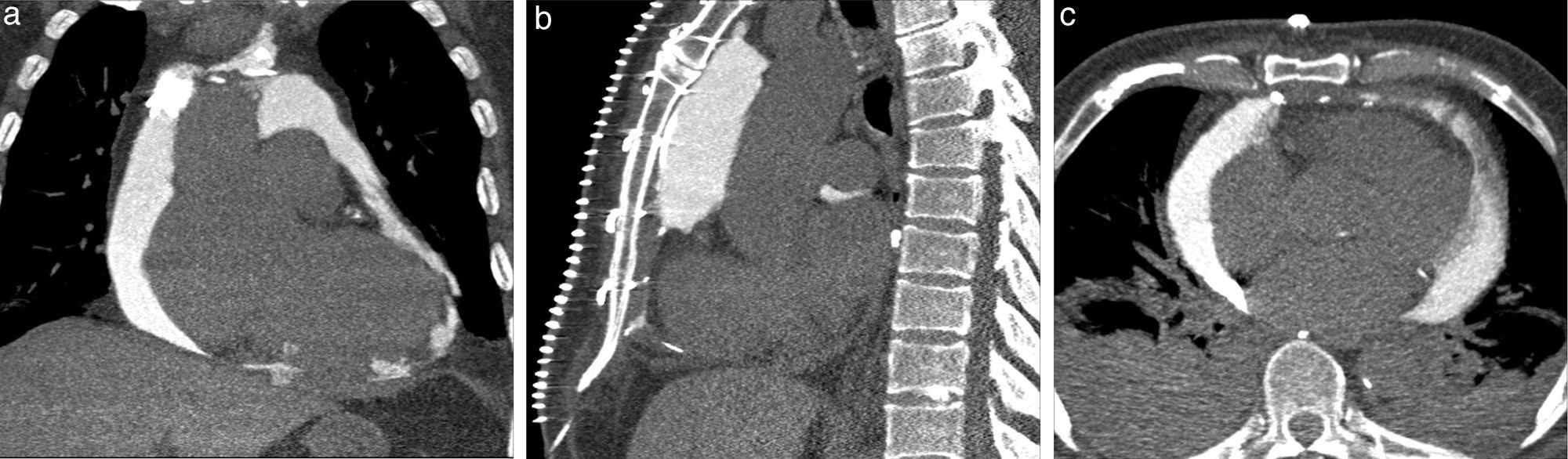
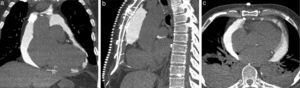
Images of the whole thorax were acquired before the procedure and were analyzed with dedicated software (Siemens Syngo Viewer®). Parameters used by protocol were: tube voltage 100 kV, tube current 110 mAs, reconstruction with 0.75 mm sections, overlap 0.5 mm. Typical window width/level used during the procedure was 700/80. The best entry point and needle orientation were determined (Figure 1). We used an 18 gauge needle from the PeriVac™ kit (Boston Scientific®). After superficial needle insertion, new images of the area of interest were acquired. Needle orientation was corrected as needed and the needle was advanced under aspiration. New images were acquired as needed. When the pericardial space was reached, a drain (8.3F pigtail catheter from the kit) was inserted through a guidewire (J-tipped 0.035″×80 cm) and final images were acquired. When in doubt (due to limited pericardial width at the puncture site) a small amount of contrast (by protocol 5 ml diluted in 10 ml of saline) was injected to confirm an intra-pericardial needle position before dilation and drain insertion (Figure 2).
Data were stored in a dedicated computer database. Statistical analysis was performed using IBM SPSS Statistics 21 (2012). Values are presented as mean ± standard deviation or median (interquartile range) as appropriate. Normality of the sample distribution was tested using the Shapiro-Wilk test.
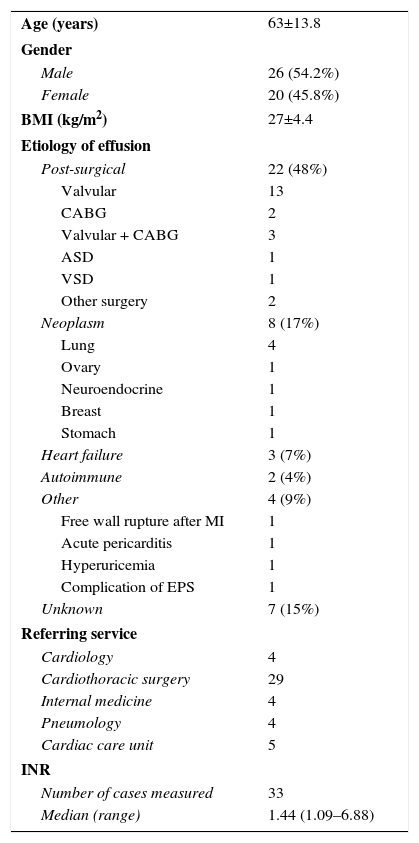
ResultsBetween August 2008 and February 2014, 49 patients were referred for CTP, which was performed in 46 patients. In three patients the effusion was considered unsuitable for pericardiocentesis (shallow or posterior location). Patient characteristics are summarized in Table 1.
Patient characteristics.
| Age (years) | 63±13.8 |
| Gender | |
| Male | 26 (54.2%) |
| Female | 20 (45.8%) |
| BMI (kg/m2) | 27±4.4 |
| Etiology of effusion | |
| Post-surgical | 22 (48%) |
| Valvular | 13 |
| CABG | 2 |
| Valvular + CABG | 3 |
| ASD | 1 |
| VSD | 1 |
| Other surgery | 2 |
| Neoplasm | 8 (17%) |
| Lung | 4 |
| Ovary | 1 |
| Neuroendocrine | 1 |
| Breast | 1 |
| Stomach | 1 |
| Heart failure | 3 (7%) |
| Autoimmune | 2 (4%) |
| Other | 4 (9%) |
| Free wall rupture after MI | 1 |
| Acute pericarditis | 1 |
| Hyperuricemia | 1 |
| Complication of EPS | 1 |
| Unknown | 7 (15%) |
| Referring service | |
| Cardiology | 4 |
| Cardiothoracic surgery | 29 |
| Internal medicine | 4 |
| Pneumology | 4 |
| Cardiac care unit | 5 |
| INR | |
| Number of cases measured | 33 |
| Median (range) | 1.44 (1.09–6.88) |
ASD: atrial septal defect; BMI: body mass index; CABG: coronary artery bypass grafting; EPS: electrophysiological study; INR: international normalized ratio; MI: myocardial infarction; VSD: ventricular septal defect.
A total of 51 procedures were reported. Five patients underwent repeat procedures due to recurrence of significant effusion (three post-surgical, two of unknown etiology). The purpose of the procedure was mainly diagnostic in only two cases. The most common etiologies of pericardial effusion were post-surgical (48%, 22 cases) and neoplasm-related (17%, eight cases).
International normalized ratio (INR) was measured in 33 procedures, including in all patients under oral anticoagulation; there were five patients with INR >2, with a maximum of 6.88.
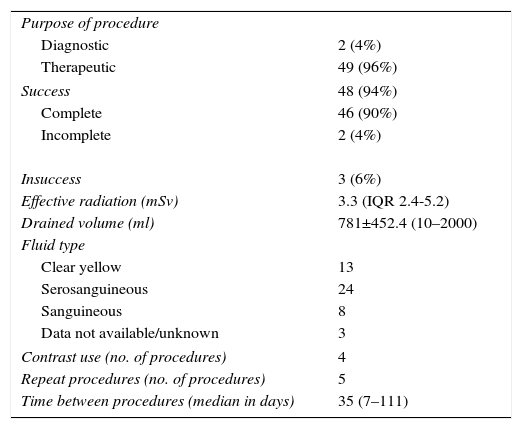
Procedural data are summarized in Table 2.
Procedural data.
| Purpose of procedure | |
| Diagnostic | 2 (4%) |
| Therapeutic | 49 (96%) |
| Success | 48 (94%) |
| Complete | 46 (90%) |
| Incomplete | 2 (4%) |
| Insuccess | 3 (6%) |
| Effective radiation (mSv) | 3.3 (IQR 2.4-5.2) |
| Drained volume (ml) | 781±452.4 (10–2000) |
| Fluid type | |
| Clear yellow | 13 |
| Serosanguineous | 24 |
| Sanguineous | 8 |
| Data not available/unknown | 3 |
| Contrast use (no. of procedures) | 4 |
| Repeat procedures (no. of procedures) | 5 |
| Time between procedures (median in days) | 35 (7–111) |
IQR: interquartile range.
The procedure had a median duration of 65 (50-80) min and median total effective radiation exposure was 3.3 (2.38–5.17) mSv.
The volume of drained fluid was variable, with a mean of 781±452.4 ml. Drainage was considered completely successful in 46 (90%) of cases. It was considered only partially successful in two cases (4%), both due to persistent fluid accumulation in a posterior location; one of them underwent successful surgical drainage, the other needed no further intervention. CTP was considered unsuccessful in three cases (6%), probably due to highly septated/organized effusions; one had been attempted for diagnostic purposes, the other two underwent successful surgical drainage.
There were no significant immediate adverse events related to the procedure, even in patients with INR >2. There was, however, one late complication, with development of pyopericardium.
The results are integrated with previously published data in Table 3.
Published series.
| Authors | Year of publication | No. of procedures | Success | Complications | |
|---|---|---|---|---|---|
| Minor | Major | ||||
| Duvernoy et al.21 | 1996 | 10 | 10 (100%) | N/A | 0 (0%) |
| Bruning et al.22 | 2002 | 11 | 11 (100%) | 0 (0%) | 1 (9%) |
| Klein et al.18 | 2005 | 319 | 314 (98.4%) | 22 (6.9%) | 1 (0.3%) |
| Palmer et al.23 | 2009 | 39 | 39 (100%) | 2 (5%) | 0 (0%) |
| Eichler et al.17 | 2010 | 20 | 20 (100%) | 0 (0%) | 0 (0%) |
| Ceviz et al.24 | 2014 | 30 | 29 (97%) | 0 (0%) | 1 (3%) |
| Current study | 2016 | 51 | 48 (94%) | 0 (0%) | 1 (2%) |
| Total: | 480 | 471 (98%) | 24 (5%) | 4 (0.8%) | |
N/A: not available.
The main finding of our study is that CT is an effective and safe technique to guide pericardiocentesis, which is an invasive and potentially dangerous procedure reserved for specific clinical scenarios.
In an era of multimodality imaging in cardiology, blind (or even ECG-guided or fluoroscopy-guided) pericardiocentesis is becoming obsolete, and is not recommendable, except in life-saving situations. Other imaging techniques may have some advantages for needle guidance. Echocardiography, above all, is a valuable and practical option, and is probably best when using a probe-mounted needle.19 It provides continuous real-time imaging, and is widely available and probably logistically simpler and less time-consuming than CT. It has, however, the disadvantage of not infrequent poor acoustic windows and less reliable needle tip visualization and orientation. CT allows the physician to better evaluate needle direction, tip positioning and relation with surrounding structures. The main limitation of CT is probably its availability, which is not as ubiquitous as echocardiography but is increasing.
CTP has previously been described as safe and effective. This series illustrates our good experience with this technique. It encompasses a reasonable-sized sample of 46 patients, almost half of them post-surgical, with a high procedural success rate (90% complete drainage, 4% incomplete and 6% unsuccessful), and no major immediate complications recorded. There was one case of pyopericardium, which developed several days after the procedure, performed for a significant effusion in the likely context of Dressler syndrome. No immediate complications were observed and the patient was transferred back to the referring hospital. He was readmitted seven days after the procedure due to recurrence of the effusion and pyopericardium was confirmed after surgical drainage. The patient eventually died 52 days after the index procedure. This was assumed to be a late complication of pericardiocentesis. Infectious complications secondary to pericardiocentesis appear to be rare but have been described before with other imaging techniques14,20 and are probably related to the drainage technique, rather than the imaging modality.
These findings of high success and low complication rates are in line with the literature. Duvernoy et al.21 reported the first series of 10 patients in 1996, all with successful drainage and no major complications. Bruning et al.22 described a series of 11 patients with effusions not drainable under sonographic surveillance, all successful, and one complication (an epicardial laceration requiring surgery). Klein et al.18 published a single-center experience of 319 procedures with a success rate of 98.4% and a major complication rate of 0.3%. In 2008 Palmer et al.23 presented a series of 39 procedures in post-surgical patients, all of them successful, without significant complications, and CTP was shown to have the additional benefit of reducing costs compared to surgical drainage. Eichler et al.17 published a series of 20 patients (85% post-surgical); all of them were successful and no major complications were reported. Ceviz et al.24 recently published a series of 30 patients, successful in 29, with one major complication (hemopericardium due to epicardial injury).
Complications are more likely to occur with increasing numbers of procedures, and are never completely avoidable in invasive procedures. Pericardiocentesis is an invasive procedure frequently performed in frail patients, and is thus associated with a non-negligible risk of complications. The decision to perform it is therefore classically conservative, given the potential benefit/risk ratio. CT guidance is proving to be an important tool in improving that ratio due to its ability to guide the procedure and increase safety. Holistic, informed, and whenever possible evidence-based clinical judgment is essential for every decision in medicine. Increasing the safety of an invasive procedure does not mean it should be performed in more patients who have smaller effusions. But it certainly means it can be performed in an urgent setting, before there is frank hemodynamic instability, which is preferable to an emergent setting, with the patient in cardiac tamponade.
In line with the published literature, this work provides evidence for the feasibility, advantages and disadvantages of CTP, when using a standardized protocol, performed by experienced physicians and in specific clinical settings.
LimitationsThis is a retrospective descriptive analysis of the experience of a single center with clinical expertise in cardiac MDCT. Additionally, this is a tertiary center with a locally available catheterization laboratory and cardiac surgery facilities. Therefore, our data reflect a specific hospital population that may preclude generalization of the findings.
ConclusionsIn our experience CTP is an effective and safe option in patients with clinical indications for pericardial fluid drainage.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.