Our study aimed to investigate the effects of alprostadil and Salvia miltiorrhiza extract on myocardial ischemia-reperfusion injury (IRI) and related underlying molecular mechanisms.
MethodsA myocardial IRI model was established in Wistar rats via surgical ligation of the left anterior descending coronary artery followed by loosening of the occlusion. The rats were divided into four groups: saline, sham, alprostadil, and S. miltiorrhiza. Rats in the saline and sham groups were injected with normal saline by tail vein once daily for 10 consecutive days. Rats in the S. miltiorrhiza and alprostadil groups were injected with S. miltiorrhiza extract (20 μg/kg) or alprostadil. Histological differences in myocardial tissues between rats in the sham group and in the myocardial IRI model were observed by hematoxylin and eosin staining. India ink perfusion was used to quantify the number of capillary microvessels. Real-time quantitative reverse transcription polymerase chain reaction was used to determine serum expression levels of soluble intercellular adhesion molecule (sICAM), soluble vascular adhesion molecule (sVCAM), CD11b and CD18.
ResultsThe alprostadil and S. miltiorrhiza groups had significantly higher numbers of microvessels than the saline group. Serum sICAM and sVCAM expression was significantly reduced in the alprostadil and S. miltiorrhiza groups. Meanwhile, sICAM and sVCAM in the alprostadil group were markedly lower than in the S. miltiorrhiza group. Moreover, the alprostadil group had markedly lower mRNA expression of CD11b and CD18, which were clearly lower than in the S. miltiorrhiza group.
ConclusionAlprostadil may have cardioprotective effects for myocardial IRI, with down-regulated expression of sICAM, sVCAM, CD11b, and CD18.
Investigar os efeitos do alprostadil e do extrato de Salvia miltiorrhiza na lesão da reperfusão da isquemia do miocárdio (RIM) e os mecanismos moleculares subjacentes relacionados.
MétodosO modelo RIM foi estabelecido através de sutura cirúrgica da artéria descendente anterior esquerda, seguida de diminuição da oclusão. Os ratos Wistar utilizados foram divididos em quatro grupos: salino, sham, alprostadil e Salvia miltiorrhiza. Os ratos do grupo salino e do grupo sham foram injetados com soro fisiológico l por veia caudal uma vez por dia durante 10 dias consecutivos. Os ratos do grupo Salvia miltiorrhiza e do grupo alprostadil foram injetados com extrato de Salvia miltiorrhiza (20 μg/kg) ou com alprostadil. As alterações histológicas nos tecidos miocárdicos entre os ratos do grupo sham e os ratos do modelo RIM foram analisadas por coloração de hematoxilina e eosina (H&E). A perfusão foi realizada para contar o número de microvasos capilares. A PCR quantitativa em tempo real foi utilizada para determinar os níveis de expressão sérica da molécula de adesão intercelular solúvel (sICAM) e da molécula de adesão vascular solúvel (sVCAM), CD11b e CD18 utilizadas.
ResultadosO grupo alprostadil e o grupo Salvia miltiorrhiza tinham um número significativamente maior de microvasos do que os ratos do grupo salino. As expressões séricas sICAM e sVCAM foram significativamente menores no grupo alprostadil e no grupo Salvia miltiorrhiza. No entanto, o sICAM e o sVCAM no grupo alprostadil foram claramente mais baixos do que os do grupo Salvia miltiorrhiza. Além disso, o grupo alprostadil revelou expressões marcadamente inferiores de mRNA de CD11b e de CD18, que foram obviamente menores do que as do grupo Salvia miltiorrhiza.
ConclusãoO alprostadil pode ter efeitos cardioprotetores na RIM, incluindo expressões down-regulated de sICAM, sVCAM, CD11b, e CD18.
Acute myocardial infarction (MI), also known as heart attack, is the major cause of mortality from cardiovascular disease worldwide.1 The main characteristic of MI is the death of myocardial cells induced by sudden occlusion or blockage of a coronary artery, leading to myocardial ischemia and infarction.2,3 It is widely acknowledged that the cardiac damage of MI is caused by ischemic and ischemia-reperfusion injury (IRI), with detrimental effects on cardiomyocytes and cardiac function.1 Therefore, investigation of myocardial IRI is an important method for studying the treatment of ischemic heart disease.
With increasing understanding of MI, various kinds of interventions have been developed, such as oxygen therapy, cell transplantation approaches, and genotype-guided antiplatelet therapy.4–7 However, mortality in MI patients remain high. Hence, more effective therapeutic strategies are urgently needed for treating MI.
Salvia miltiorrhiza is used in traditional Chinese medicine, alone or in combination with other traditional Chinese medicines that have favorable effects in many diseases, such as metabolic disorders, cardiovascular disease and malignant tumors.8,9 Huang et al. reported that S. miltiorrhiza and ligustrazine injection significantly improved cardiac function in a myocardial IRI rat model.10
Alprostadil, whose main component is prostaglandin E1, has a wide range of pharmacological effects, including expanding blood vessels, suppressing platelet aggregation, and promoting deformation of red blood cells, thereby improving microcirculation.11 Prostaglandin E1 infusion is an accepted therapy for critical congenital heart disease, particularly in low-birth-weight newborns, and lack of prostaglandin E1 is associated with higher morbidity in infants with congenital heart disease.12,13 According to a study by Liu et al., administration of alprostadil for 14 weeks markedly attenuated increased serum total cholesterol, triglyceride, and low-density lipoprotein cholesterol levels in rats with coronary heart disease on a high-cholesterol diet, suggesting that alprostadil has cardiac protective effects.14 Injection of liposomal prostaglandin E1 before occlusion of the left anterior descending coronary artery (LAD) until reperfusion for 1 h in a porcine model significantly elevated left ventricular systolic pressure and end-diastolic pressure, and marked reductions in myocardial no-reflow area, serum interleukin-6 and tumor necrosis factor-alpha levels were observed.15 Moreover, it has been reported that myocardial infarct size and indices of myocardial function (such as creatine kinase isoenzyme, lactate dehydrogenase, and troponin T) were markedly reduced by intravenous administration of alprostadil in rats, while levels of superoxide dismutase, catalase, nitric oxide and phosphorylated endothelial nitric oxide synthase 3 in the left ventricle were increased.16 Although the above studies indicated the protective effects of alprostadil against myocardial IRI, these effects have not been compared with those of S. miltiorrhiza and the related underlying molecular mechanisms have not been clearly revealed.
In our study, a myocardial IRI model was created in rats by surgical ligation of the LAD. Rats in the alprostadil and S. miltiorrhiza groups were injected with alprostadil or S. miltiorrhiza by tail vein once daily for 10 consecutive days. The effects were assessed by observations of histopathological changes and microvascular patency. At the same time, expression levels of soluble adhesion molecules in serum were measured by real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR).
MethodsAnimals and experimental groupsTwenty-six Wistar rats (weight: 180-220 g; male/female: 1/1) were used in our study. A myocardial IRI rat model was induced according to the surgical method described by Xu et al.,17 in which after anesthesia, 18 rats underwent surgical ligation of the LAD for about 30 min to induce ischemic injury. The slipknot was then loosened to cause reperfusion injury. Rats in the sham group (n=8) were subjected to the same operation without LAD ligation. Following occlusion surgery, the 18 rats were randomly divided into three groups (n=6 per group) as follows: saline, alprostadil, and S. miltiorrhiza. The dosage of 20 μg/kg alprostadil was selected based on the results of a pilot study in our laboratory (data not shown) and the study by Erer et al.18 Rats in the alprostadil group were injected with alprostadil (Beijing Ted Pharmaceutical Co., Ltd, China) by tail vein at doses of 20 μg/kg once daily for 10 consecutive days. Rats in the saline and sham groups were injected with an equal volume of normal saline by tail vein once daily for 10 consecutive days. Rats in the S. miltiorrhiza group were injected with S. miltiorrhiza extract (20 μg/kg) once daily for 10 consecutive days.
The animal experimental protocols were approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (number: SYDW2020-003) and conducted in strict accordance with the standards of the Guide for the Care and Use of Laboratory Animals published by the Ministry of Science and Technology of the People's Republic of China.
Histopathological examinationMyocardial tissues were excised, rinsed in saline and fixed in 10% formalin solution for 48 h at room temperature. The tissues were then dehydrated and embedded in paraffin. The paraffin-embedded tissues were cut into serial sections of 5 μm thickness for hematoxylin and eosin staining using a commercial kit (Besso Biotechnology Co., Ltd., Zhuhai, China). Histological differences in myocardial tissues between rats in the sham group and in the myocardial IRI model were observed under light microscopy (Olympus).
India ink perfusionIndia ink perfusion was used to quantify the number of capillary microvessels after 10 days of drug administration. Perfusion consisted of an initial flush phase to wash out all blood elements and a subsequent tracer-perfusion phase.19 Firstly, the left ventricle was rapidly cannulated and perfused by normal saline solution at a pressure of 90-110 mmHg until colorless effluent was observed. Afterwards, ink plus dextran (ink/dextran: 7/3) was injected into the heart at room temperature (25-30°C). When the limbs turned dark gray, the great vessels of the cardiac base and the superior and inferior vena cava were ligated. The hearts were subsequently resected and maintained at 4°C for at least 1 h, removed and fixed in 4% paraformaldehyde. Subsequently, cryosectioning was carried out and observed under light microscopy.
Real-time quantitative reverse transcription polymerase chain reactionTotal RNA was extracted from blood samples after 10 days of drug administration using an RNA extraction kit (Laifeng Biotech Co., Ltd, China), according to the manufacturer's instructions. The isolated RNA was reverse transcribed into cDNA using a First Strand cDNA Synthesis Kit (Fermentas Life Science, Canada). Briefly, 1 μg total RNA and 11 μl diethyl pyrocarbonate (DEPC)-treated water were mixed on ice, incubated at 65°C for 5 min, and put on ice. The reaction mixture consisted of 4.1 μl 5× reaction buffer, 0.6 μl RiboLock™ RNase inhibitor (20 U/μl), 2.1 μl deoxynucleotide (dNTP) mix (10 mM), 1.1 μl Reverse Tra Ace, 1.1 μl Oligo (dT) primer (0.5 μg/μl) and 11 μl DEPC-treated water were added and incubated at 42°C for 60 min, followed by termination of the reaction at 72°C for 10 min.
The reverse transcription reaction products were used in PCR applications. The total reaction volume was 20 μl, including 10 μl SYBR Green PCR Mix (Synergy Brands), 1 μl forward primer, 5 μl reverse primer and 4 μl sterilized distilled water containing cDNA. The primer sequences are listed in Table 1. RT-qPCR was conducted on a 7900HT Fast Real-Time PCR system (Applied Biosystems, USA) according to the following reaction conditions: pre-denaturation at 95°C for 2 min; 40 cycles at 95°C for 15 s, 60°C for 20 s and 72°C for 20 s; a melting curve at 60-95°C for 30 s with a heating rate of 0.5°C/s. Measurements of soluble intercellular adhesion molecule (sICAM), soluble vascular adhesion molecule (sVCAM), CD11b and CD18 expression were normalized using beta actin (ACTB) as an internal control in each sample. Relative gene expression levels were calculated using the 2-ΔΔCT method. Data were obtained from three independent experiments performed in triplicate.
Primer sequences for quantitative real-time reverse transcription polymerase chain reaction.
| Gene | Primer sequence (5′-3′) | Length in bp |
|---|---|---|
| sICAM | F: 5′-GCCTGGGGTTGGAGACTAAC-3′ | 91 |
| R: 5′-CTGTCTTCCCCAATGTCGCT-3′ | ||
| sVCAM | F: 5′-CTGTTGACATCTCCCCTGGAT-3′ | 200 |
| R: 5′-AGAGTGCTCATCCTCAACACC-3′ | ||
| CD11b | F: 5′-GTGCTGAACTGCTCTGTTGC-3′ | 281 |
| R: 5′-CTGCCCACAATGAGTGGTACA-3′ | ||
| CD18 | F: 5′-CCTGCAGATTGTTCCGGAGT-3′ | 88 |
| R: 5′-CTGGGGCCACCTTTACTGAG-3′ | ||
| ACTB | F: 5′-AGGCTGTGTTGTCCCTGTATG-3′ | 203 |
| R: 5′-AGCTGTGGTGGTGAAGCTGTA-3′ |
bp: base pairs; F: forward; R: reverse.
Data are presented as mean ± standard deviation. Statistical analyses were performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). After the normality of the data was determined, analysis of variance (ANOVA) was used to compare data with normal distribution in multiple groups and the Kruskal-Wallis test for data with non-normal distribution. Comparisons between experimental and control groups were performed using the Student's t test. A p-value <0.05 was considered statistically significant.
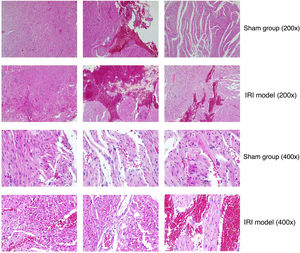
ResultsHistopathological changesAfter IRI, obvious myocardial damage, focal loss of striatum, focal attenuation of sarcoplasm, vasodilation and congestion were observed in myocardial tissues of myocardial IRI model rats (Figure 1). Myocardial fibers were necrotic and their outline was unclear. In addition, myocytes were partially dissolved and muscle fibers were broken, suggesting IRI due to myocardial infarction.
Comparison of myocardial tissues from rats in the sham group and rats of the myocardial ischemia-reperfusion injury (IRI) model. Representative images are shown at 200× and 400× magnification (hematoxylin and eosin staining). In specimens from myocardial IRI rats, the ischemic region showed focal attenuation of sarcoplasm and focal loss of striations. Hyperemic blood vessels and extravasation of erythrocytes were observed in muscle fibers in the sham group to a lesser extent than in the myocardial IRI model.
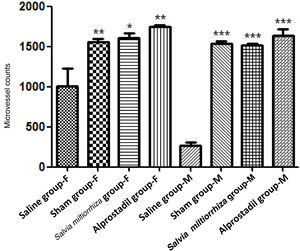
Using ink perfusion, the number of microvessels was quantified for rats in each group (Figure 2). For female rats, the numbers of microvessels were 1011±220, 1552±89, 1612±93, and 1747±58 in the saline, sham, alprostadil, and S. miltiorrhiza groups, respectively. There was a significant difference in the numbers of microvessels among these groups in female rats by ANOVA (p<0.001, Table 2). Meanwhile, the numbers of microvessels were 269±86, 1537±48, 1520±34, 1635±144 in male rats in the saline, sham, alprostadil, and S. miltiorrhiza groups, respectively. Significant differences were also observed in the numbers of microvessels among these groups in male rats by ANOVA (p<0.001) (Table 2). The numbers of microvessels were significantly different between male and female rats in the saline group (p=0.008) (Table 3). Both female and male rats presented significantly greater numbers of microvessels in the sham, alprostadil, and S. miltiorrhiza groups compared with the saline group (p<0.05) (Figure 3).
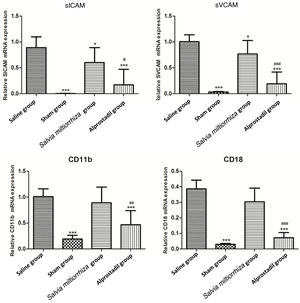
ANOVA revealed significant differences in sICAM (p<0.001), sVCAM (p<0.001), CD11b (p<0.001), and CD18b (p<0.001) between the saline, sham, Salvia miltiorrhiza, and alprostadil groups (Table 4). Compared with the saline group, mRNA expression of sICAM and sVCAM were significantly reduced in the sham, alprostadil, and S. miltiorrhiza groups (p<0.05) (Figure 4). mRNA expression of sICAM and sVCAM in the alprostadil group was markedly lower than in the S. miltiorrhiza group (p<0.05).
Comparisons of soluble intercellular adhesion molecule, soluble vascular adhesion molecule, CD11b and CD18 mRNA expression levels between rats in the saline, sham, Salvia miltiorrhiza, and alprostadil groups.
| Factors | F | p |
|---|---|---|
| sICAM | 25.721 | <0.001 |
| sVCAM | 56.134 | <0.001 |
| CD11b | 29.535 | <0.001 |
| CD18b | 70.589 | <0.001 |
sICAM: soluble intercellular adhesion molecule; sVCAM: soluble vascular adhesion molecule.
Real-time quantitative reverse transcription polymerase chain reaction for soluble intercellular adhesion molecule (sICAM), soluble vascular adhesion molecule (sVCAM), CD11b and CD18 mRNA expression levels of rats in the saline, sham, Salvia miltiorrhiza, and alprostadil groups. *: p<0.05 compared with saline group; **: p<0.01 compared with saline group; ***: p<0.001 compared with saline group; #: p<0.05 compared with S. miltiorrhiza group; ##: p<0.01 compared with S. miltiorrhiza group; ###: p<0.001 compared with S. miltiorrhiza group.
The sham and alprostadil groups had markedly lower mRNA expression of CD11b and CD18 than the saline group (p<0.05). However, no significant difference was observed in mRNA expression of CD11b and CD18 between the saline and S. miltiorrhiza groups. Furthermore, mRNA expression of CD11b and CD18 in the alprostadil group was also markedly lower than in the S. miltiorrhiza group (p<0.05).
DiscussionMyocardial infarction is caused by ischemia due to occlusion of the coronary arteries, which when followed by reperfusion of the myocardium results in IRI, a complicated process involving immunological and metabolic factors.20 Focal stenosis, diffuse epicardial atherosclerosis, and microvascular coronary artery disease are different between females and males.21,22 Gender differences have been observed in renin response and changes in capillary diameter after renal IRI.23 In our study, the numbers of microvessels were compared in male and female rats. There were significant differences in the numbers of microvessels between male and female rats in the saline group, which underwent occlusion surgery. Both male and female rats in the sham, alprostadil, and S. miltiorrhiza groups had significantly higher numbers of microvessels than rats in the saline group. Alprostadil might be a potential therapy for preventing microvascular IRI based on the above results.
RT-qPCR revealed that serum sICAM and sVCAM expression was significantly reduced in the sham, alprostadil, and S. miltiorrhiza groups. Furthermore, sICAM and sVCAM expression in the alprostadil group was markedly lower than in the S. miltiorrhiza group. There is increasing evidence indicating that IRI-induced microvascular dysfunction is largely due to leukocyte-endothelial cell interactions.24,25 Leukocytes migrate to the area of injury followed by adhesion to endothelial cells.26 sVCAM, the soluble form of vascular cell adhesion molecule, is mainly expressed in activated endothelium and mediates recruitment of leukocytes in the inflammatory process, while sICAM, a circulating form of intercellular adhesion molecule (also known as CD54), is the counter receptor for lymphocyte function-associated antigen, which contributes to leukocyte adhesion and migration through endothelial cells.27 Significantly elevated serum sICAM and sVCAM levels have been observed in patients with old cerebrovascular ischemic stroke and chronic brain infarction.28,29 It has been reported that resveratrol significantly reduces myocardial infarct size and improves postischemic ventricular function in myocardial IRI rats, with lower sICAM and sVCAM levels.30 Therefore, attenuation of IRI in myocardial infarction due to alprostadil could be associated with the down-regulation of sICAM and sVCAM expression, inhibiting leukocyte adhesion and infiltration in injured tissues.
Moreover, rats in the sham and alprostadil groups had markedly lower mRNA expression of CD11b and CD18, while mRNA expression of CD11b and CD18 in the alprostadil group was also lower than in the S. miltiorrhiza group. According to Wang et al., total salvianolic acid, the major water-soluble ingredient of S. miltiorrhiza, improves microcirculatory disturbance caused by IRI, with lower production of oxygen free radicals in the venular wall and CD11b/CD18 expression on neutrophils.31 Activation of neutrophils by oxygen free radicals and proinflammatory mediators released after IRI increases the expression of adhesion molecules on both neutrophils and the endothelium, which recruits neutrophils to the vascular endothelial surface to which they adhere, followed by transendothelial migration and interaction with myocytes.32 A study by Liu et al. revealed that microRNA-141 mimics have cardioprotective effects, as evidenced by reductions in serum parameters of myocardial function (cardiac troponin I and lactate dehydrogenase) and decreased infarct size in myocardial IRI mice, with decreased cardiac ICAM-1 expression and CD11b+ cell and F4/80+ macrophage infiltration into ischemic myocardial tissue.33 Previous studies have revealed that injection of anti-CD18 antibodies before reperfusion can markedly reduce myocardial infarct size and protect left ventricular function after reperfusion.34,35 Thus, alprostadil may ameliorate IRI in myocardial infarction associated with the down-regulation of CD11b and CD18.
ConclusionsThe alprostadil and S. miltiorrhiza groups had significantly higher numbers of microvessels than the saline group. At the same time, serum sICAM and sVCAM expression was significantly reduced in the alprostadil and S. miltiorrhiza groups, while sICAM and sVCAM expression in the alprostadil group was markedly lower than in the S. miltiorrhiza group. Moreover, the alprostadil group had markedly lower mRNA expression of CD11b and CD18, which in turn was lower than in the S. miltiorrhiza group. Alprostadil may have cardioprotective effects against myocardial IRI, associated with down-regulation of sICAM, sVCAM, CD11b, and CD18.
Ethics approval and consent to participateThe animal experimental protocols were approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University and conducted in strict accordance with the standards of the Guide for the Care and Use of Laboratory Animals published by the Ministry of Science and Technology of the People's Republic of China.
Funding2016 Harbin Application Technology Research and Development Project Planning Task (Contract) Book [2016RAQYJ207].
Authors’ contributionsJing Liu and JingBo Hou conceived, designed and performed the experiments. HuiBo Chen, WeiZhi Sun and XiaoXia Jin analyzed and interpreted the data. Wei Zhang, YanBo Yang, YaRu Li and XiuLi Chen contributed methods, materials, analysis tools or data. Jing Liu wrote the paper. All authors read and approved the final manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
Not applicable.