A 69-year-old woman with idiopathic dilated cardiomyopathy and chronic heart failure experienced repeated hospital admissions for acute pulmonary edema with no recognizable precipitating factor. Worsening mitral regurgitation was triggered by exercise echocardiography and significant intraventricular dyssynchrony was elicited by low-dose dobutamine stress echocardiography. After cardiac resynchronization therapy she remained free of hospitalizations for 12 months. This case highlights the dynamic nature of both functional mitral regurgitation and left ventricular dyssynchrony and illustrates how in some patients stress echocardiography can help to clarify clinical scenarios and help with the challenging task of selecting patients who will benefit from cardiac resynchronization therapy.
Doente do sexo feminino, 69 anos de idade, com miocardiopatia dilatada idiopática e insuficiência cardíaca, que teve múltiplos internamentos por edema agudo do pulmão sem causa precipitante aparente. A demonstração do agravamento da regurgitação mitral no ecocardiograma de esforço e o aparecimento de uma dissincronia intraventricular óbvia, para além de reserva contráctil, no eco de stress com dobutamina, foram decisivos para a orientação terapêutica. A doente foi submetida a terapêutica de ressincronização cardíaca e manteve-se sem hospitalizações durante 12 meses. Este caso realça o carácter dinâmico da regurgitação mitral funcional e da dissincronia ventricular esquerda. Ilustra, ainda, como a ecocardiografia de stress pode ajudar, em alguns doentes, a esclarecer quadros clínicos e ajudar na difícil tarefa que é selecionar doentes que irão beneficiar da terapêutica de ressincronização cardíaca.
Readmission rates are extremely high in patients with chronic heart failure (CHF). Identification of the pathophysiologic mechanism implicated in the acute deterioration of compensated CHF is essential to target treatment accordingly and prevent further readmissions.1 Functional mitral regurgitation (FMR) is, by nature, dynamic and its evaluation by echocardiography may require a stress evaluation, which can also expose latent mechanical dyssynchrony in failing hearts.2,3 Through these attributes, stress echocardiography can assist in clarifying puzzling clinical scenarios as well as in defining the therapeutic strategy for the individual patient with relapsing acute heart failure symptoms.
Case reportA 69-year-old woman with known idiopathic dilated cardiomyopathy and chronic heart failure (CHF) was admitted after an episode of decompensation presenting as acute pulmonary edema. Over the previous four years since the diagnosis was first established, she had been admitted on several occasions for decompensated heart failure for which known precipitating factors were identified: hypertensive crisis, paroxysmal atrial fibrillation and poor compliance with medication. In the three months preceding the current admission, she experienced four successive episodes of pulmonary edema, all with no recognizable precipitating factor.
Her medical history revealed a long history of treated hypertension and dyslipidemia, and she had been followed in NYHA class II, under optimal medical therapy including beta-blockers, ACE inhibitors, furosemide, spironolactone, nitrates and warfarin, in addition to amiodarone and a statin. Eighteen months before the current admission, she received an implantable cardioverter-defibrillator (ICD) for primary prevention. Left ventricular ejection fraction was 30%.
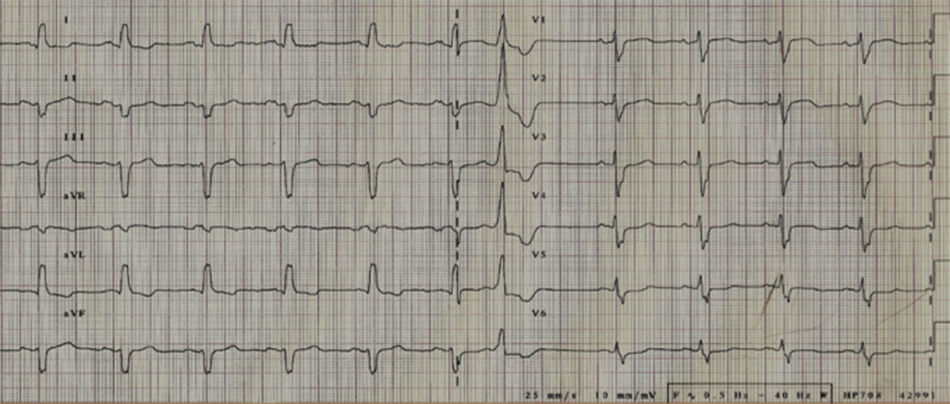
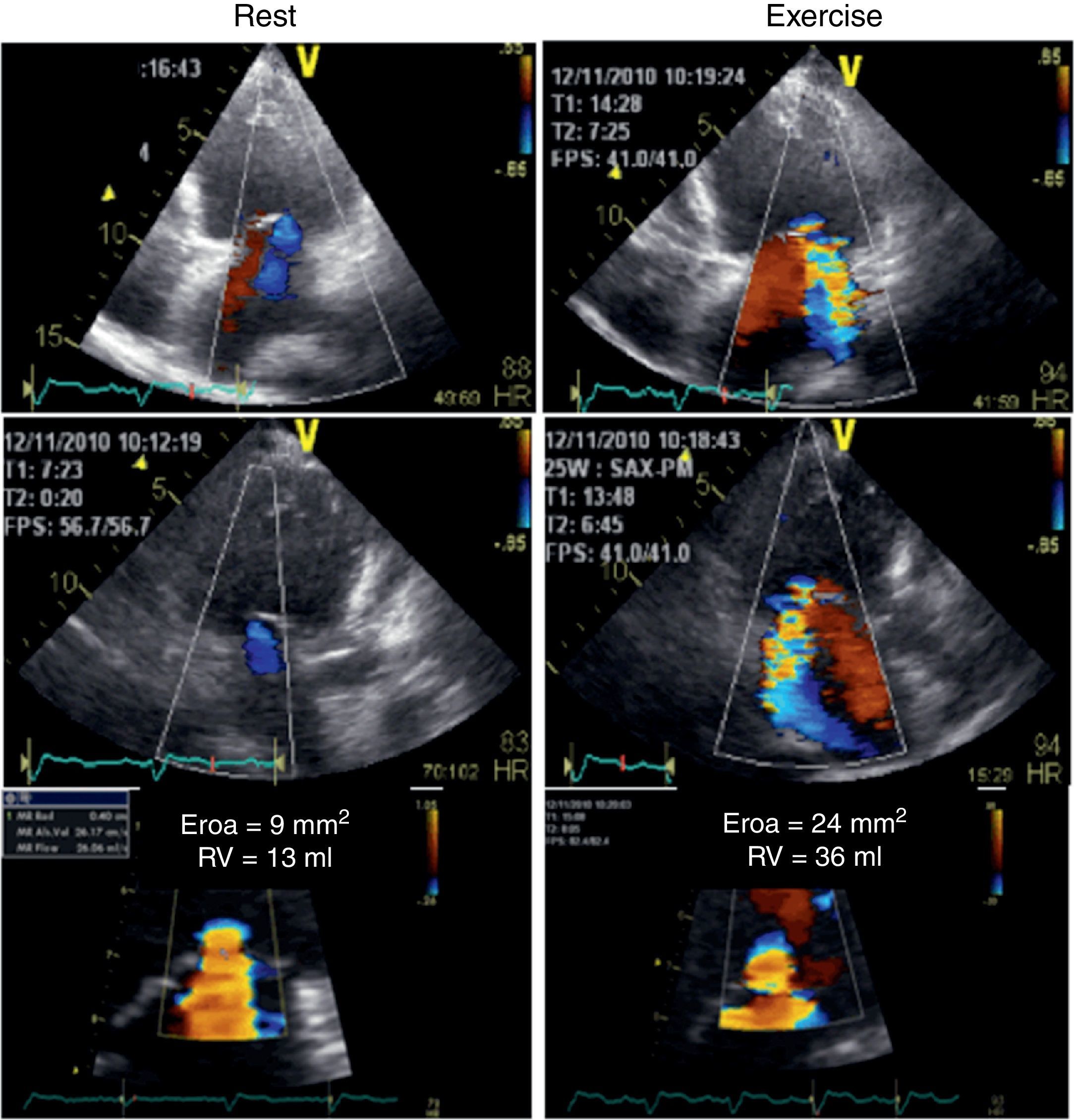
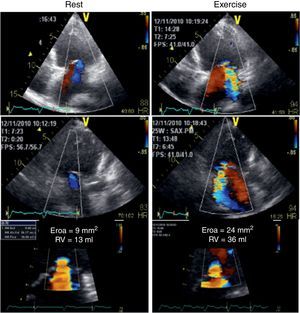
Her ECG showed sinus rhythm with an intraventricular conduction delay (QRS duration 143 ms) with no obvious left bundle branch block (Figure 1). The echocardiogram showed a spherical dilated left ventricle with 32% ejection fraction (sphericity index 0.83; end-diastolic volume 117 ml; end-systolic volume 79 ml) and a moderately dilated left atrium (indexed end-systolic volume 37 ml/m2). The left ventricular filling pattern was consistent with delayed relaxation (E/A <1) and an E/e’ ratio of 12. The mitral valve presented a tenting pattern, causing mild regurgitation at rest; effective regurgitant orifice area (EROA) was 9 mm2 and regurgitant volume (RV) was 13 ml by the PISA method. Atrioventricular dyssynchrony was absent, but there was interventricular dyssynchrony, with (Q-Ao) − (Q-Pulm) = 62 ms, and borderline intraventricular dyssynchrony (130 ms delay between septal and posterior walls by two-dimensional speckle tracking radial strain), with a faint septal flash. After clinical stabilization, a symptom-limited bicycle stress test was performed under full therapy on an echo tilt table. The patient exercised for six minutes at a low workload (20–50 W), interrupted due to severe dyspnea. Her blood pressure rose from 120/67 mmHg to 160/84 mmHg and her heart rate from 68 to 96 beats per minute. No left ventricular segmental dyssynergies, arrhythmias or ST-segment changes were induced. Nevertheless, an obvious increase in mitral regurgitation during exercise was observed, with an increase of 15 mm2 (from 9 to 24 mm2) in EROA and an increase of 23 ml (from 13 to 36 ml) in RV (Figure 2). However, this worsening was not considered sufficient to justify surgical correction, especially in view of several echocardiographic morphological parameters predicting unsuccessful repair. Furthermore, the indication for cardiac resynchronization therapy (CRT) was questionable, given the non-LBBB QRS morphology and the QRS duration of <150 ms.
Color flow Doppler echocardiogram and flow convergence proximal to the effective regurgitant orifice at rest (left) and at peak exercise (right). The left panel demonstrates mild functional mitral regurgitation while the right panel shows the increase in mitral regurgitation following exercise, becoming moderate.
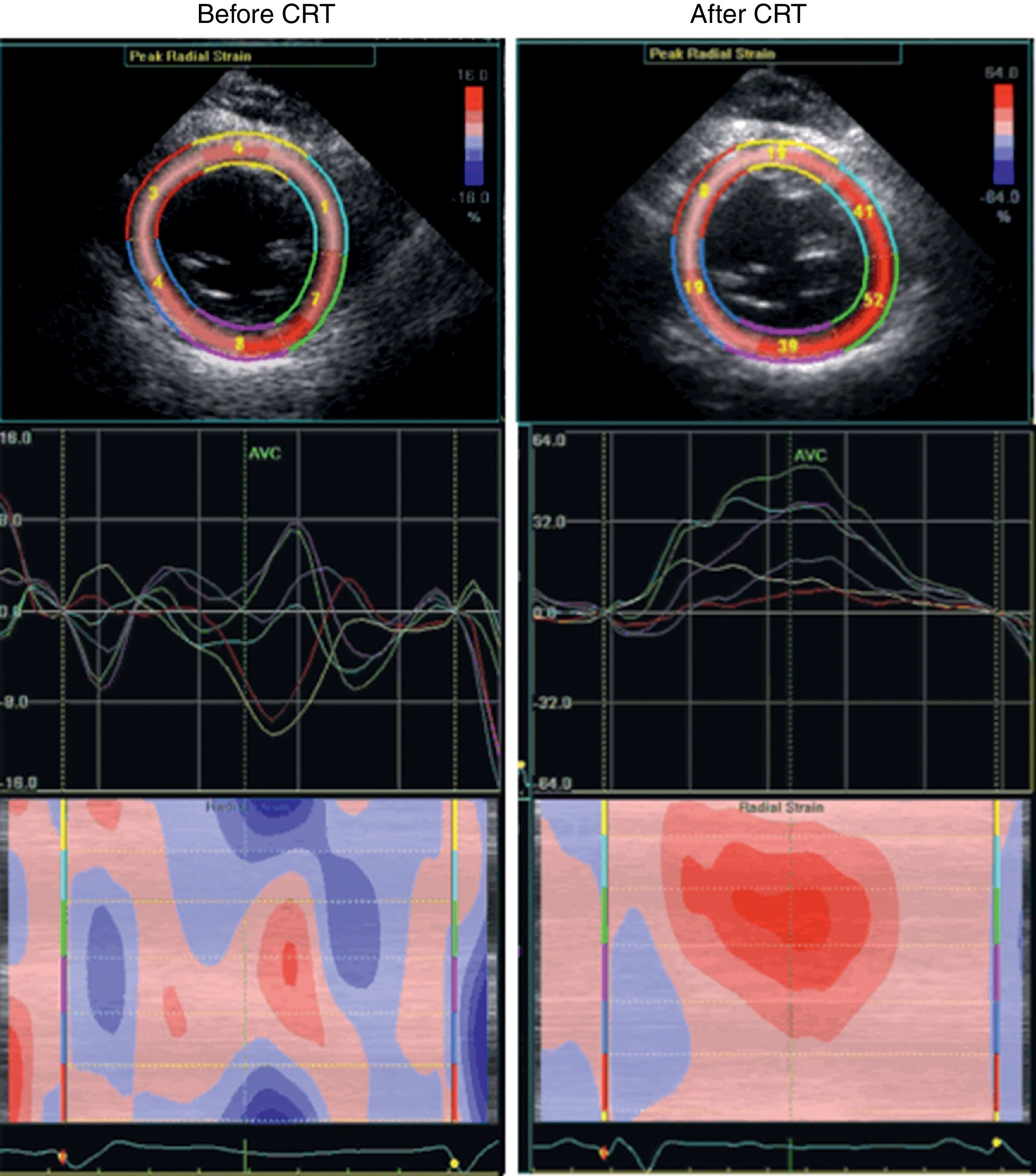
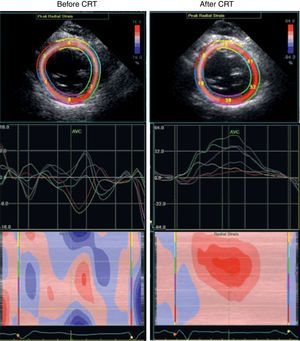
Taking all this into account, we decided to pursue our investigation through a low-dose dobutamine stress echo (5–20 μg/kg/min). At peak dobutamine dose, intraventricular dyssynchrony became evident with an obvious septal flash, despite an improvement in global contractility, attesting the presence of contractile reserve. At this point, the resynchronization option seemed the most appropriate and the patient's ICD was upgraded to a cardiac resynchronization therapy device with defibrillator function. Three months later echocardiography showed no significant change in LV volume, ejection fraction, or mitral regurgitation at rest, but there was a clear improvement in cardiac output (from 2.9 to 4.6 l/min) and in ventricular synchronicity (Figure 3). The patient remained free of hospitalizations for 12 months.
Discussion and ConclusionIn patients with CHF readmitted for acute decompensation, efforts should be made to identify correctable causes or precipitating factors, as well as to ensure an optimal volume status and a tailored and titrated oral pharmacological regimen prior to discharge. However, in our patient, compliance with this approach and a close follow-up failed to prevent consecutive early readmissions, even on the day of discharge. Accordingly, we felt that something more had to be done.
Functional mitral regurgitation (FMR) is common in patients with CHF and LV dysfunction, regardless of etiology. FMR results from an imbalance between closing and opening forces on the mitral leaflets and has a negative impact on both symptoms and prognosis.4,5 In clinical practice, echocardiography is usually performed at rest. Several investigators have described dynamic changes in FMR, mainly exercise-induced, in patients with LV dysfunction. In patients with ischemic dilated cardiomyopathy and recent acute pulmonary edema, Piérard et al. showed that, unlike in patients in a control group, regurgitant volume doubled during exercise Doppler echocardiography, with increasing pulmonary pressures and limiting dyspnea.6 Exercise-induced changes in the severity of FMR in ischemic patients have been related to indices of valve deformation, but not to induction of myocardial ischemia.7 Also in non-ischemic patients, a significant increase in mitral valve EROA may be associated with dynamic LV dyssynchrony unmasked by exercise, as shown by D’Andrea et al. in a series of 60 patients with idiopathic dilated cardiomyopathy and narrow QRS duration undergoing supine bicycle exercise echocardiography.8 Moreover, several authors have reported the prognostic value of exercise-induced changes in severity of mitral regurgitation.9,10
In our patient, we were able to demonstrate not only a substantial increase in mitral valve EROA and RV during exercise, but also an increase in LV dyssynchrony induced by low-dose dobutamine stress echocardiography. The coexistence of dynamic mitral regurgitation and dynamic left ventricular dyssynchrony has been reported in more than one-third of patients with chronic systolic LV dysfunction and is strongly correlated with changes in stroke volume during exercise, causing symptoms out of proportion to LV dysfunction and resting MR.11,12
How best to select patients who will benefit from CRT is still a matter of debate. A significant lack of consistency between QRS duration and CRT response is recognized. Several recent observational studies have underlined the association between FMR and LV electromechanical delay in dilated patients with prolonged QRS.13 Moreover, patients showing contractile reserve with dobutamine have a favorable clinical and reverse remodeling response to CRT.14–16
This case confirms that both FMR and LV synchronicity have a dynamic component. It also supports the concept that exercise is the preferred stressor for the assessment of dynamic mitral regurgitation, while both exercise and dobutamine can be used in the assessment of LV dyssynchrony. Finally, it highlights the role of stress echocardiography for clarifying unforeseen clinical scenarios as well as helping to carry out the challenging task of selecting CRT candidates.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.