Heart failure (HF) remains a prevalent syndrome with significant morbidity and mortality. Optimal drug and device therapies are crucial to reduce the risk of death or HF admission. Yet, less symptomatic patients with good functional capacity are often perceived as having a low risk of adverse events and their attending physicians may suffer from prescription inertia or refrain from performing therapy optimization. Maximum or peak oxygen consumption (pVO2) assessed during cardiopulmonary exercise testing (CPET) is often used as a prognosis indicator and surrogate marker for functional capacity. Our goal was to assess clinical outcomes in a seemingly low risk HF population in Weber class A (pVO2>20 mL/kg/min) with reduced left ventricular ejection fraction (LVEF).
MethodsSingle-center retrospective observational study enrolling consecutive HF patients with LVEF<40% (HFrEF) performing CPET between 2003 and 2018. Those with pVO2 >20 mL/kg/min were included. The primary endpoint was a composite of all-cause death or HF hospitalizations at two years after CPET. We also assessed the rates of N-terminal pro b-type natriuretic peptide (NT-proBNP) elevations at baseline.
ResultsSeventy-two patients were included (mean age of 53±10 years; 86% male; 90% NYHA I-II; median LVEF 32%; median pVO2 24 mL/kg/min). At baseline, 93% had an NT-proBNP level >125 pg/mL (median NT-proBNP 388 [201–684] pg/mL). Overall, seven patients (10%) met the primary endpoint: three died (4%) and five (7%) had at least one HF admission. Among those who died, only one patient had an HF admission during follow up.
ConclusionIn a clinically stable HFrEF population with good functional capacity, persistent neurohormonal activation was present in the majority, and one in ten patients died or had a HF admission at two years’ follow-up. These findings support the urgent need to motivate clinicians to pursue optimal drug uptitration even in less symptomatic patients.
A insuficiência cardíaca (IC) com fração de ejeção reduzida é uma síndrome prevalente com morbimortalidade significativa. O aprimoramento da terapêutica é crucial para reduzir o risco de morte ou admissão por IC. No entanto, doentes paucissintomáticos com boa capacidade funcional são frequentemente considerados como tendo um baixo risco de eventos adversos, não são, por isso, alvo de aprimoramento terapêutico. O consumo máximo de oxigénio (pVO2), avaliado durante provas de esforço cardiorrespiratórias (PECR), é utilizado como um marcador prognóstico e da capacidade funcional do doente. O nosso objetivo foi avaliar o número e tipo de eventos clínicos numa população de IC com aparente baixo risco em classe Weber A (pVO2 > 20 mL/kg/min) e fração de ejeção ventricular esquerda (FEVE) reduzida.
MétodosEstudo observacional, retrospetivo, unicêntrico, incluindo doentes consecutivos com IC com FEVE <40% (ICFEr) que realizaram PECR entre 2003-2018. Doentes com pVO2 >20 mL/kg/min foram incluídos. O endpoint primário foi um composto de morte por todas as causas ou hospitalização por IC nos dois anos após a PECR. Também avaliámos a percentagem de doentes com elevação basal de NT-proBNP.
ResultadosForam incluídos 72 doentes (idade 53±10 anos; 86% homens; 90% classe NYHA I-II; FEVE média 32%; pVO2 mediana 24 mL/kg/min), 93% apresentavamm um nível de NT-proBNP >125 pg/mL (NT-proBNP mediano 388 [201-684] pg/mL). Globalmente, sete doentes (10%) atingiram o endpoint primário: três por óbito (4%) e cinco (7%) tiveram pelo menos uma hospitalização por IC. Entre os doentes que faleceram, apenas um teve uma hospitalização por IC, pelo que contabilizado pelo primeiro evento (hospitalização).
ConclusãoNuma população clinicamente estável de doentes com ICFEr e boa capacidade funcional, a maioria apresentou ativação neuro-hormonal persistente e um em cada dez atingiu o endpoint primário (óbito ou internamento por IC) durante um follow-up de dois anos após a PECR. Esses achados fortalecem a necessidade urgente de promover a prescrição otimizada de terapêuticas modificadoras de doença.
Heart failure (HF) is a chronic syndrome with an ever-increasing prevalence and high risk of mortality, hospitalizations and disability, particularly for patients not receiving optimal guideline-directed medical therapy (GDMT).1,2 Accurate risk stratification is a key step in HF assessment, and misperception of risk can lead to suboptimal care.3 Physicians managing HF patients are more easily prompted to modify the therapy in those with New York Heart Association (NYHA) class III or IV symptoms than in those who are asymptomatic or have a no more than mild limitation of functional capacity.3 Whereas the former are expected to have a progressively worsening condition, characterized by recurrent hospitalizations and high short to intermediate term mortality, the latter are perceived to have a low risk of adverse outcomes.3 Consequently, physicians will often refrain from optimizing drugs and doses, or implanting devices in HF with reduced ejection fraction (HFrEF) patients that are deemed clinically stable and in NYHA class II. This decision is taken to avoid the risks of side effects and the increased burden of multiple healthcare encounters.4
Yet, HFrEF should be viewed as a progressive disease in patients with a significant mass of at-risk viable myocardium, in whom myocardial dysfunction is amenable to improvement with optimal GDMT.5 Neurohormonal activation, which is initially compensatory, never switches off in HF.5 These systems are constantly on in an attempt to compensate for the failing heart's inability to maintain normal cardiovascular homeostasis. The chronic presence of these circulating neurohormones exacerbates hemodynamic abnormalities, which encourages further remodeling, neurohormone release and additional hemodynamic deterioration.
To illustrate the dire prognosis displayed in HFrEF patients, even if only mildly symptomatic, we assessed the rates of N-terminal pro b-type natriuretic peptide (NTproBNP) elevation and major adverse outcomes in an HFrEF population with good functional capacity, defined by a maximum oxygen consumption (pVO2) higher than 20 mL/kg/min.
Population, materials and methodsStudy population and data collectionThis is a single-center retrospective cohort study enrolling consecutive HFrEF patients undergoing cardiopulmonary exercise testing (CPET) in our center between January 2003 and December 2018. Those who had a peak O2 uptake higher than 20 mL/kg/min were included. HFrEF was defined as per European Society of Cardiology 2016 guidelines.1 All patients underwent left ventricle ejection fraction (LVEF) evaluation via transthoracic echocardiography using biplane Simpson method at our center within a six-month window before CPET.
Maximal symptom-limited cardiopulmonary exercise testing (CPET) was performed and several parameters were assessed, including pVO2, percent of predicted pVO2 (determined by the Wasserman equation6), minute ventilation/carbon dioxide output (VE/VCO2) slope, exercise oscillatory ventilation (EOV) and respiratory exchange ratio (RER), defined as the oxygen consumption/carbon dioxide consumption ratio. Subjects were encouraged to exercise until RER was ≥1.10, as per site protocol. Twelve-lead electrocardiogram, blood pressure and pulse oximetry were monitored during CPET. Gas exchange was continuously assessed by face mask and determined by MedicalGraphics® and Carefusion® gas analyzer. Peak O2 consumption was taken as the highest 20-second averaged VO2 during the exercise period of CPET. Twenty-second averaged minute ventilation and carbon dioxide output, from the initiation to the end of exercise, were used to calculate the VE/VCO2 slope. EOV was defined as an oscillatory pattern persisting for ≥60% of the test at an amplitude of ≥15% of the average resting value.7
Demographic and clinical data, as well as laboratory assessments were obtained from the electronic medical records. Laboratory evaluation (including NT-proBNP) was performed within seven days before CPET, as per center protocol.
OutcomesThe primary clinical endpoint was a composite of all-cause death or HF hospitalization, whichever occurred first, at two years follow up after CPET. HF hospitalization was defined as an admission for acute HF management.8 Sensitivity analysis included defining a good functional capacity using a predicted pVO2 ≥60% according to previous recommendations.9
Statistical analysisContinuous variables were expressed as mean±standard deviation (SD) (normal distribution) or median and interquartile range (IQR) (non-parametric). Time-to-event analysis was performed by applying Kaplan-Meier curves. Categorical variables were expressed as counts (percentage). Statistical analysis was performed using Statistical Package for the Social Sciences Statistics v.15.0 (IBM, New York, USA).
ResultsStudy populationOverall, 407 HF patients performed CPET between 2003 and 2018, of which 72 were included (70 patients excluded due to LVEF >40%; 265 patients excluded due to pVO2 equal to or lower than 20 mL/kg/min). Baseline demographics are depicted in Table 1. Mean age was 52±10 years, 83% patients were male, most patients (90%) had NYHA functional class I–II symptoms, and 46% had ischemic HF. Median LVEF was 32% (IQR: 27–35%), mean systolic blood pressure (SBP) was 110±17 mmHg (36% with systolic blood pressure <110 mmHg) and mean heart rate (HR) was 70±10 bpm. With respect to GDMT, 94% patients were on renin-angiotensin-system inhibitors (RAASi), 90% on beta-blockers, 42% on mineralocorticoid receptor antagonists (MRA), and 50% were prescribed loop diuretics. A total of 32 patients (44%) had an implantable cardioverter-defibrillator and/or a cardiac resynchronization therapy device.
Patients’ baseline demographics.
| Baseline demographics | Overall cohort(N=72) |
|---|---|
| Age – years, mean±SD | 51.6±9.8 |
| Male sex – n (%) | 60 (83.3) |
| BMI – kg/m2, median (IQR) | 26.5 (25.0–28.7) |
| HF etiology – n (%) | |
| Ischemic | 33 (45.8) |
| Valvular | 3 (4.2) |
| Other | 36 (50.0) |
| LVEF – percentage, median (IQR) | 32.0 (27.0–35.0) |
| NYHA classification – n (%) | |
| I | 18 (25) |
| II | 47 (65.3) |
| III | 7 (9.7) |
| Mean systolic blood pressure – mmHg, mean±SD | 110±17 |
| Systolic blood pressure<90 mmHg | 4 (6) |
| Systolic blood pressure<100 mmHg | 14 (20) |
| Systolic blood pressure<110 mmHg | 24 (36) |
| Mean heart rate – beats per minute, mean±SD | 70±10 |
| Laboratory data | |
| Hemoglobin – g/dL, mean±SD | 14.2±1.4 |
| Serum sodium – mmol/L, mean±SD | 140.0±4.4 |
| eGFR (MDRD) – mL/min/1.73 m2, mean±SD | 92.7±23.3 |
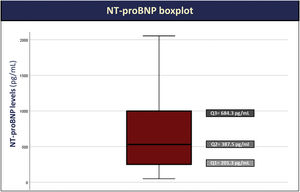
| NT-proBNP – pg/mL, median (IQR) | 388 (201–684) |
| NT-proBNP≥125 pg/mL – n (%) | 67 (93.1) |
| NT-proBNP≥600 pg/mL – n (%) | 31 (43.1) |
| Comorbidities – n (%) | |
| Hypertension | 27 (37.5) |
| Diabetes mellitus | 9 (12.5) |
| AF | 8 (11.1) |
| CKD (eGFR<60 mL/min/1.73 m2by MDRD equation) | 32 (45.1) |
| Medications – n (%) | |
| Beta-blockers | 65 (90.3) |
| RAASi* | 68 (94.4) |
| MRA | 30 (41.7) |
| Loop diuretics | 36 (50.0) |
| Devices – n (%) | |
| ICD | 18 (25.0) |
| CRT-D | 14 (19.4) |
| CPET variable | |
| pVO2– mL/kg/min, median (IQR) | 23.5 (21.3–25.4) |
| Percent of predicted pVO2– percentage, median (IQR) | 84.7 (74.4–91.4) |
| VE/VCO2slope – L/min, median (IQR) | 31.5 (27.1–38.9) |
| EOV –n (%) | 16 (22.9) |
| RER | 1.10 (1.08–1.19) |
| >1.10, n (%) | 54 (75) |
| >1.05, n (%) | 68 (94) |
AF: atrial fibrillation; CKD: chronic kidney disease; CPET: cardiopulmonary exercise testing; CRT: cardiac resynchronization therapy; eGFR: estimated glomerular filtration rate; EOV: exercise oscillatory ventilation; HF: heart failure; HFSS: Heart Failure Survival Score; ICD: implantable cardioverter defibrillator; IQR: interquartile range; LVEF: left ventricle ejection fraction; MECKI: Metabolic Exercise test data combined with Cardiac and Kidney Indexes; MRA: mineralocorticoid receptor antagonist; NYHA: New York Heart Association; pVO2: peak O2 consumption; RER: respiratory exchange ratio; SD: standard deviation; VE/VCO2: minute ventilation/carbon dioxide production.
Cardiopulmonary exercise testing results are illustrated in Table 1. Patients had a median pVO2 of 24 mL/kg/min (IQR 21–25 mL/kg/min), a median percentage of predicted pVO2 of 85% (interquartile range (IQR) 74–91%) and a median VE/VCO2 slope of 32 (IQR 27–39). Overall, 75% had a RER >1.10 and 23% had EOV.
The majority of patients had an NT-proBNP >125 pg/mL (93%), with median NT-proBNP levels of 388 pg/mL (IQR: 201–684 pg/mL) (Figure 1).
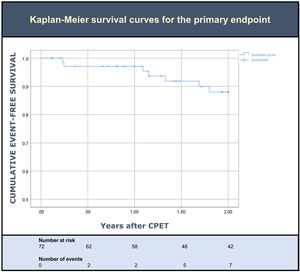
Clinical outcomesDuring the two years of follow up after CPET, seven patients (10%) met the primary outcome. Overall, three died (4%) and five (7%) had at least one HF hospitalization (Table 2). Death was preceded by a HF hospitalization in only one of the three patients. An event-free survival curve analysis for the primary endpoint is shown in Figure 2. Sensitivity analysis using a cut-off of predicted pVO2 ≥60% resulted in a similar rate of events.
In a real-world single-center HFrEF cohort, one in ten patients with good functional capacity diagnosed by CPET had major clinical events over a two-year period of follow up. More than 90% had persistent NT-proBNP elevations.
Heart failure remains a high risk disease with a grim short-term prognosis. Our study cohort consisted of patients receiving appropriate drug therapy (>90% BB and RAASi), who seemed clinically well (good functional capacity and half not requiring a loop diuretic). Nevertheless, 10% died or had at least one HF hospitalization within two years. Similar observations were previously reported in the PARADIGM-HF (Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure) trial. Among the least symptomatic patients in NYHA class I or II, 22% either died from a cardiovascular cause or had at least one HF hospitalization during a median follow up of 27 months.10 Furthermore, among the more clinically stable patients without any history of HF hospitalization, 20% either died from a cardiovascular cause or had at least one HF hospitalization during the course of the trial. The primary event was CV death with no preceding HF hospitalization in 51% of these most clinically stable patients, therefore precluding patients and physicians from an alert that could have prompted treatment intensification and optimization to avoid this dismal outcome.11 Overall, 60% of those CV deaths were sudden cardiac deaths.11 In our study, death was preceded by a HF hospitalization in only one of three patients.
In HFrEF, evidence of subclinical disease progression can be found even in patients who seem to be doing well and to be receiving appropriate treatment. Neurohormonal activation persisted in the majority of our study patients, although exercise tolerance, as judged by NHYA class, was normal or only mildly impaired in >90%. Additional evidence of this illusion of clinical stability derives from studies using troponin measurements where the majority of stable HFrEF patients in NYHA class II have chronic cardiac troponin elevations, corroborating the subclinical continuous myocardial lesion.12 Several mechanisms can lead to troponin elevation in HF including supply-demand mismatch, which may occur in the absence of coronary obstruction due to increased oxygen demand related to increased wall tension, anemia, or other factors provoking subendocardial injury. Non-coronary triggers include cellular necrosis or apoptosis in the context of wall stress, as well as the toxic effects of circulating neurohormones, toxins, inflammation, and infiltrative processes. Regardless of the mechanism, troponin elevation in HF is associated with greater tendency toward LV remodeling, and a higher risk of death or hospitalization.12,13 Recent evidence indicates that changes in NT-proBNP levels are predictive of similar directed changes in troponin levels, which in turn are associated with left ventricular forward remodeling.14 Nevertheless, in our population, we had only access to baseline NT-proBNP levels, and serial assessments of cardiac biomarkers were beyond the scope of this study.
On the basis of the above-mentioned observations, GDMT uptitration to maximum tolerated doses or maximum tested doses is paramount and currently recommended in European Society of Cardiology HF guidelines.1 However, the aforementioned illusion of stability may be hazardous and lead to treatment inertia, particularly when dealing with chronic individuals not overwhelmingly symptomatic. Adherence to GDMT recommendations by physicians has traditionally been shown to be suboptimal and clinical stability may be one of the culprit reasons.15,16 Indeed, less than one in five patients admitted for acute HF and discharged on beta-blockers had these uptitrated in the following month.16 These observations account for a reduced number of HFrEF patients achieving GDMT-target doses as demonstrated in the IMPROVE-HF registry.5
Such inertia is particularly important in stable, pauci-symptomatic patients, where misperception of risk and concerns regarding side effects may deter clinicians and patients from appropriate treatment intensification.3 Yet, evidence shows that even less symptomatic, NYHA class II patients benefit from appropriate GDMT. Indeed, such cases often account for a significant number (if not the majority) of patients included in landmark HF trials.17–20 Moreover, the benefits of GDMT in less symptomatic patients are at least similar in magnitude to those observed in more symptomatic patients, as shown in pre-specified subgroup analyses of PARADIGM-HF and HEAAL (effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure) trials.18,20
Our findings strengthen previous recommendations: GDMT initiation and uptitration is essential, since even clinically stable, ‘low risk’ patients, with good functional capacity have persistent neurohormonal activation and a significant rate of events. Accordingly, viewing chronic HF as a stable disease may be a misnomer and, therefore, must be avoided.
This study has some limitations. First, it is a single-center retrospective study with a limited sample size. Second, we did not assess other important adverse events related to HF, namely cardiovascular death, malignant arrythmias or implantable cardioverter defibrillator shocks. Third, we did not assess whether GDMT was being taken at maximum recommended dose. However, in order to estimate if our cohort was close to (or distant from) optimal GDMT we assessed patients’ SBP and HR as surrogates – assuming that those presenting a low SBP/HR would not tolerate further up-titration of renin-angiotensin-aldosterone inhibitors and/or beta-blockers. Only 36% of patients had a systolic blood pressure <110 mmHg. Although it may be possible that some patients with higher SBP and HR were still in the process of up-titration, already on maximal doses of disease-modifying drugs or unable to reach these because of intolerance or other limitations (e.g., kidney failure or hyperkalemia), it seems reasonable to consider that a significant proportion of the patients in our cohort would be indeed amenable to further drug up-titration. Fourth, included patients performed an echocardiographic assessment within six months of CPET. We cannot exclude a possible worsening or improvement of LGEF within this time frame.
ConclusionIn a clinically stable HFrEF population displaying preserved functional capacity, persistent neurohormonal activation was present in the majority and one in ten patients died or had one HF admission at two years post-CPET. These findings rightfully challenge the perception that treatment optimization may be less important for low-symptomatic patients. Uptitration of pharmacological therapy to maximal tolerated or tested doses is mandatory to slow disease progression as effectively as possible, even when the clinical scenario seems (deceptively) favorable. Therefore, strategies are urgently needed to motivate clinicians to pursue GDMT even in such patients.
Authors’ contributionsSM wrote the first draft of this article; CB, GJL, BR, and FG incorporated feedback in subsequent drafts and revisions; CS, PF, AD, AT, AV, CA and MM contributed to revisions and reviewed the final draft; SM composed the figures and submitted the final version of this article, on behalf of all the authors. All named authors complied with the International Committee of Medical Journal Editors guidelines for authorship.
Ethical approvalThis investigation was conducted in accordance with the World Medical Association Declaration of Helsinki (seventh revision, Fortaleza, 2013) and the Declaration of Istanbul (2008). This study was exempt from ethical approval.
FundingNone declared.
Conflicts of interestThe authors have no conflicts of interest to declare.