Chronic thromboembolic pulmonary hypertension (CTEPH) is a progressive, but potentially curable, form of pulmonary hypertension. Pulmonary endarterectomy (PEA) is a complex surgery that frequently achieves hemodynamic normalization and symptom resolution, although not all patients are suitable for the procedure. We aimed to assess long-term outcomes of CTEPH, namely all-cause mortality and hospital admission for decompensated heart failure, according to treatment modalities in patients who underwent PEA or non-surgical therapy.
MethodsA 10-year retrospective study of patients with CTEPH at a referral center was conducted. Forty-five patients were included and median follow-up time was 57 (IQR 24-93) months. Survival analysis was performed and a multivariate Cox regression model was used to identify independent predictors of outcomes.
ResultsPatients were mostly female (59%) and mean age was 63±16 years. Two-thirds were severely symptomatic at diagnosis, with 62.2% of patients presenting in WHO functional class (WHO FC) III or IV. One-, two- and three-year survival was 93.3%, 82.4% and 75.9%, respectively. Serum BNP (HR 1.003; 95% CI: 1.001-1.005; p=0.003) and creatinine (HR 12.092; 95% CI: 1.121-130.390; p=0.040) were predictors of death. Mortality was numerically lower in those who underwent PEA (p=0.135). PEA was associated with decreased risk of the combined endpoint of all-cause mortality and hospital admission for decompensated heart failure (HR 0.198; 95% CI: 0.040-0.982; p=0.047), as were lower serum BNP (HR 1.003; 95% CI: 1.001-1.005; p=0.008) and mPAP (HR 1.073; 95% CI: 1.022-1.128; p=0.005) at diagnosis. Most patients who underwent PEA presented improved WHO FC (92.9%) and post-surgical residual pulmonary hypertension was identified in only 21.4%.
ConclusionPEA provided a better overall prognosis than non-surgical therapy, improving symptoms and frequently achieving hemodynamic normalization, with a numerical trend for lower mortality. Higher serum BNP, creatinine and mPAP at diagnosis were independently associated with worse outcomes.
A hipertensão pulmonar tromboembólica crónica (HPTEC) é uma forma progressiva, mas potencialmente curável, de hipertensão pulmonar. A endarterectomia pulmonar (EAP) é uma cirurgia complexa que frequentemente alcança a normalização hemodinâmica e resolução sintomática, embora nem todos os doentes sejam candidatos ao procedimento. O objetivo foi avaliar o prognóstico dos doentes com HPTEC submetidos a EAP ou a terapêutica não cirúrgica.
MétodosEstudo retrospetivo dos doentes com HPTEC num centro de referência durante 10 anos. O tempo mediano de seguimento foi 57 (IQR 24-93) meses. Foi realizada análise de sobrevivência e utilizado um modelo de regressão de Cox para identificar preditores independentes de prognóstico.
ResultadosForam incluídos 45 doentes, maioritariamente mulheres (59%), com 63±16 anos. Ao diagnóstico, 62,2% dos doentes encontravam-se nas classes III ou IV da OMS. A sobrevivência a 1, 2 e 3 anos foi 93,3%, 82,4% e 75,9%, respetivamente. O BNP (HR 1,003;95%CI[1,001-1,005]; p=0,003) e a creatinina (HR 12,092;95%CI[1,121-130,390]; p=0,040) foram preditores de mortalidade. A mortalidade foi numericamente inferior no grupo da EAP (p=0,135). A EAP associou-se a menor risco de ocorrência do endpoint combinado de mortalidade e admissão hospitalar por insuficiência cardíaca descompensada (HR 0,198;95%CI[0,040-0,982]; p=0,047), tal como menores valores de BNP (HR 1,003;95%CI[1,001-1,005]; p=0,008) e pressão arterial pulmonar média (HR 1,073;95%CI [1,022-1,128]; p=0,005). A maioria dos doentes operados apresentou melhoria da classe funcional (92,9%) e hipertensão pulmonar residual foi identificada em apenas 21,4%.
ConclusãoA EAP providenciou melhor prognóstico que a terapêutica não cirúrgica, associando-se a melhoria sintomática e frequente normalização hemodinâmica, com tendência numérica para menor mortalidade.
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare disease characterized by thrombotic and remodeling changes in pulmonary vessels resulting in a progressive, but potentially curable, form of pulmonary hypertension (PH). The condition affects 3.2-4.0% of patients who survive pulmonary embolism (PE),1–3 although it is diagnosed without a prior history of an acute event in 25.2% of cases.4 The pathophysiology of CTEPH involves occlusive organized thromboembolic material causing mechanical arterial obstruction as well as microvascular remodeling. The precise mechanisms leading to vascular remodeling are still unclear, but dysfunctional fibrinolysis, bronchopulmonary venous shunting, endothelial dysfunction, inflammatory mechanisms and genetic susceptibility appear to play a role.5,6 The molecular mechanisms of small-vessel disease partially overlap with those of pulmonary arterial hypertension (PAH).7 These changes ultimately lead to increased pulmonary vascular resistance (PVR), increased pulmonary arterial pressure and, eventually, right heart failure. The symptoms are mostly related to progressive right ventricular dysfunction, and are thus non-specific and initially present only on exertion, making early diagnosis challenging.8 As a result, a median delay of 14.1 months between initial symptoms and diagnosis has been reported4 and concerns about underdiagnosis have been raised.9 Diagnosis is obtained by precise measurement of pulmonary arterial pressures and evidence of vascular obstruction and/or perfusion defects.10
The treatment of choice for operable CTEPH is pulmonary endarterectomy (PEA). This is a complex surgical procedure with non-negligible perioperative mortality that frequently achieves normalization of hemodynamics and long-term symptomatic benefit.11,12 Despite being a potential cure for CTEPH, PEA is not suitable for all patients; only 63-68% of patients are technically eligible for PEA,4,13 as extensive involvement of the distal vessels may not be surgically accessible and discordance between PVR and surgically accessible obstructions may indicate extensive secondary vasculopathy that is not treatable by this approach.14 Underlying comorbidities and high surgical risk may also preclude the procedure, as well as patients’ personal preference, resulting in reported surgical rates of 50-58%.4,13,15
Two treatment modalities are available for inoperable CTEPH (as well as residual or recurrent PH after PEA): targeted therapy with pulmonary vasodilators, and balloon pulmonary angioplasty (BPA). Riociguat, a soluble guanylate cyclase stimulator, is the sole targeted therapy approved for CTEPH, improving hemodynamics and providing sustained benefits in exercise and functional capacity.16–18 However, off-label use of drugs approved for PAH may also be justified.10,19,20 BPA is an emerging treatment option in which small balloons are used to open obstructed vessels and widen stenotic lesions, over multiple sessions. It provides hemodynamic, functional and symptomatic benefits to inoperable patients.21,22
The available therapies for CTEPH are not mutually exclusive and can be used in combination,14,23 and the most suitable treatment should be decided in a multidisciplinary team setting.10,14
ObjectivesThe aims of this study were to characterize a cohort of CTEPH patients followed in a referral center, to assess long-term outcomes according to the chosen treatment modality, and to identify predictors of mortality and morbidity.
MethodsStudy designWe designed a single-center, retrospective study enrolling all patients diagnosed with CTEPH at the Pulmonary Vascular Unit of Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal, between January 2009 and December 2018. CTEPH was defined by precapillary PH confirmed by right heart catheterization in the presence of diagnostic signs for CTEPH on computed tomography pulmonary angiography (CTPA), single-photon emission computed tomography (SPECT) and/or conventional catheter-based pulmonary angiography, after three months of effective anticoagulation with a vitamin K antagonist. Precapillary PH was defined by mean pulmonary arterial pressure (mPAP) ≥25 mmHg with pulmonary artery wedge pressure (PAWP) ≤15 mmHg.10
Data were collected regarding demographics, previous history of PE and risk factors, and clinical, laboratory, imaging, and hemodynamic findings at diagnosis, as well as the decision of the multidisciplinary team regarding operability and the proposed treatment approach.
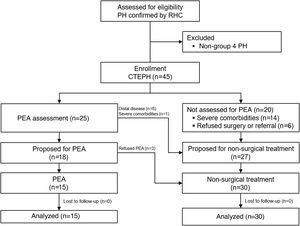
For the analysis, patients were divided into two groups: group 1, composed of patients who initially underwent PEA; and group 2, composed of patients who initially received non-surgical treatment (Figure 1).
The study followed the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of Centro Hospitalar e Universitário de Coimbra.
Treatment decision and operability assessmentAfter the diagnosis of CTEPH was established, the patients were referred to a PEA center for multidisciplinary assessment of operability. Patients were operated in two different PEA centers: most procedures (12) were conducted in a foreign expert center that performs more than 50 procedures annually with a mortality rate below 5%,14 while the other three underwent PEA at a national center that performs fewer than 50 procedures annually.24
Follow-up and outcomesAll follow-up visits were conducted at the Pulmonary Vascular Unit. Treatment at the last hospital visit was recorded, as recommendations for medical therapy were revised and its availability changed during the study period. Postoperative hemodynamic assessment was performed at least 12 months after PEA. Median follow-up time was 57 (IQR 24-93) months, and all patients were followed for at least one year, excluding censoring due to death. Two- and three-year follow-up was completed in 76% and 64% of patients, respectively.
The primary endpoint was all-cause mortality. The secondary endpoint was a combination of all-cause mortality and hospital admission for decompensated heart failure.
Statistical analysisData analysis was conducted using IBM SPSS Statistics version 23.0. Regarding descriptive statistics, means with standard deviation or medians with IQR were used to characterize the distribution of continuous variables. Absolute frequencies and percentages were used to describe categorical variables. A paired-samples t test was performed to compare hemodynamics at diagnosis and after PEA.
Kaplan-Meier curves were used for survival analysis. Entry date was defined as the first day of treatment (either the date of surgery or the first day of pharmacological treatment). Patients alive at the end of the study were censored. Comparison between the two groups was performed using a log-rank test.
Univariate Cox proportional hazards analysis was performed to identify possible predictors of outcomes. These variables, along with those previously reported as related to mortality for CTEPH, were then included in a forward stepwise multivariate Cox regression model to identify independent predictors of the primary and secondary endpoints.
ResultsClinical characteristicsDuring the 10-year period, 45 patients were consecutively included. The patients’ clinical and hemodynamic characteristics are presented in Table 1. Mean age was 62.5±16.3 (IQR 48.5-76.0) years and patients were predominantly female (57.8%). Most (n=34) had no previous history of symptomatic venous thromboembolism. At diagnosis, almost two-thirds were moderately to severely symptomatic: 62.2% were in World Health Organization functional class (WHO FC) III or IV and there were significant hemodynamic alterations (Table 1).
Clinical and hemodynamic data at diagnosis.
| Total (n=45) | Surgical (n=15) | Non-surgical (n=30) | p | |
|---|---|---|---|---|
| Female, n (%) | 26 (57.8) | 8 (53.3) | 18 (60.0) | 0.670 |
| Age, years | 62.5±16.3 | 53.9±14.7 | 66.8±15.5 | 0.011 |
| History of acute PE, n (%) | 16 (35.6) | 5 (33.3) | 11 (36.7) | 0.826 |
| WHO FC III-IV, n (%) | 28 (62.2) | 11 (73.3) | 17 (56.7) | 0.277 |
| I, n (%) | 2 (4.4) | 1 (6.7) | 1 (3.3) | |
| II, n (%) | 15 (33.3) | 3 (20.0) | 12 (40.0) | |
| III, n (%) | 23 (51.1) | 11 (73.3) | 12 (40.0) | |
| IV, n (%) | 5 (11.1) | 0 (0) | 5 (16.7) | |
| BNP, pg/ml | 323.9±397.3 | 259.0±231.6 | 368.2±479.4 | 0.419 |
| Creatinine, mg/dl | 1.0±0.3 | 1.00±0.26 | 1.03±0.31 | 0.726 |
| Hemoglobin, g/dl | 14.8±1.7 | 15.2±1.2 | 14.7±1.8 | 0.325 |
| Hemodynamics | ||||
| RAP, mmHg | 8.9±4.4 | 9.7±5.3 | 8.5±3.9 | 0.400 |
| mPAP, mmHg | 48.7±12.6 | 49.3±8.6 | 48.3±14.4 | 0.801 |
| PAWP, mmHg | 11.2±4.9 | 11.7±5.9 | 10.9±4.3 | 0.612 |
| PVR, UW | 11.3±4.7 | 11.4±4.4 | 11.3±5.0 | 0.908 |
| CO, l/min | 3.6±1.4 | 3.9±2.2 | 3.4±0.9 | 0.336 |
| CI, l/min/m2 | 2.1±0.8 | 2.2±1.3 | 2.0±0.4 | 0.367 |
| SvO2, % | 62.6±8.4 | 64.4±9.5 | 61.6±7.8 | 0.332 |
| 6MWT, m | 364.3±128.9 | 424.5±77.8 | 331.9±140.2 | 0.028 |
6MWT: six-minute walk test; BNP: type B natriuretic peptide; CI: cardiac index; CO: cardiac output; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary artery wedge pressure; PE: pulmonary embolism; PVR: pulmonary vascular resistance; RAP: right atrial pressure; SvO2: mixed venous oxygen saturation; WHO FC: World Health Organization functional class.
CTEPH was mostly identified by CTPA or lung ventilation/perfusion SPECT. The latter presented better sensitivity (100%) in our cohort than CTPA (68.6%) and confirmed the diagnosis after a negative CTPA in 11 patients. Although 86.7% of patients underwent catheter-based pulmonary angiography to confirm the diagnosis and to assess operability, only one patient was diagnosed exclusively by this method.
TreatmentA total of 25 patients were referred to a PEA center for multidisciplinary treatment decision. Eighteen patients (72%) were considered operable (three of whom refused surgery) and seven (28%) were considered inoperable due to predominantly distal disease (n=6) or severe comorbidities resulting in an unacceptable surgical risk (n=1). The other 20 patients were not referred for multidisciplinary assessment at a PEA center, due to comorbidities and/or marked frailty (n=14) or refusal of surgical treatment and/or referral to another country (n=6).
Patients selected for surgical treatment were younger and achieved longer distances in the six-minute walk test (6MWT) than those proposed for medical therapy, with no other significant difference between the two groups. Median time from diagnosis to PEA was seven (IQR 4.0-9.5) months. Only two of the patients proposed for PEA began pulmonary vasodilator therapy while awaiting surgery.
Two patients (7%) from the non-surgical group underwent BPA, while the remainder underwent exclusively pharmacological therapy. Two patients (7%) from this group were later proposed for surgical treatment and were awaiting PEA at the end of the follow-up period. Most patients who underwent PEA did not require pulmonary vasodilator therapy during follow-up (75%). At the last hospital visit, 38% were being treated with monotherapy and 33% were on combination therapy (Table 2). Regarding anticoagulation, most patients were on a vitamin K antagonist (80%), with the other 20% on direct oral anticoagulants.
Medical therapy at last hospital visit.
| Total (n=45) | Surgical (n=15) | Non-surgical (n=30) | |
|---|---|---|---|
| Monotherapy, n (%) | 17 (37.8) | 0 | 17 (56.7) |
| PDE5i | 9 (20.0) | 0 | 9 (30.0) |
| Riociguat | 7 (15.6) | 0 | 7 (23.3) |
| ETA | 1 (2.2) | 0 | 1 (3.3) |
| Combination therapy, n (%) | 15 (33.3) | 3 (20.0) | 12 (40.0) |
| PDE5i+ERA | 7 (15.6) | 0 | 7 (23.3) |
| Riociguat+ERA | 6 (13.3) | 2 (13.3) | 4 (13.3) |
| PDE5i+prostanoid | 1 (2.2) | 0 | 1 (3.3) |
| PDE5i+ERA+prostanoid | 1 (2.2) | 1 (6.7) | 0 |
| No pulmonary vasodilator therapy, n (%) | 13 (28.9) | 12 (80.0) | 1 (3.3) |
| No therapy | 12 (26.7) | 12 (80.0) | 0 |
| Best supportive care | 1 (2.2) | 0 | 1 (3.3) |
PDE5i: phosphodiesterase 5 inhibitor; ERA: endothelin receptor antagonist. Best supportive care was defined as appropriate palliative care without pulmonary vasodilator therapy.
During follow-up 11 patients died (24.4%), two (13.3%) in the PEA group and nine (30.0%) of those selected for pharmacological treatment. One-, two- and three-year survival was 93.3%, 82.4% and 75.9%, respectively. Kaplan-Meier curves for overall survival are presented in Figure 2. Although mortality was numerically higher in those proposed for pharmacological therapy, this did not achieve statistical significance (log-rank p=0.135; hazard ratio [HR] 0.327). However, after one perioperative death was excluded, PEA was associated with lower all-cause mortality (log-rank p=0.046; HR 0.158).
Regarding the secondary endpoint, the surgical approach was associated with a better prognosis, as shown in Figure 2 (log-rank p=0.036; HR 0.232). Two patients (13.3%) were hospitalized for decompensated heart failure or died, fewer than in the non-surgical group (n=13; 43.3%).
Serum BNP (HR 1.003; 95% confidence interval [CI] 1.001-1.005; p=0.003) and creatinine (HR 12.092; 95% CI: 1.121-130.390; p=0.040) at diagnosis were independently associated with the primary endpoint using a multivariate Cox regression model (Table 3). Regarding the secondary endpoint, PEA was associated with better outcomes (HR 0.198; 95% CI: 0.040-0.982; p=0.047), and BNP (HR 1.003; 95% CI: 1.001-1.005; p=0.008) and mPAP (HR 1.073; 95% CI: 1.022-1.128; p=0.005) at diagnosis were identified as independent predictors of the event.
Multivariate analysis for predictors of the primary and secondary endpoints.
| Variable | HR (95% CI) | p | |
|---|---|---|---|
| Primary endpoint | BNP (pg/ml) | 1.003 (1.001-1.005) | 0.003 |
| Creatinine (mg/dl) | 12.092 (1.121-130.390) | 0.040 | |
| Secondary endpoint | BNP (pg/ml) | 1.003 (1.001-1.005) | 0.008 |
| mPAP (mmHg) | 1.073 (1.022-1.128) | 0.005 | |
| PEA | 0.198 (0.040-0.982) | 0.047 |
The Cox proportional hazards model included as variables age, gender, history of acute pulmonary embolism, WHO functional class III/IV, BNP, serum creatinine, distance in six-minute walk test, mPAP, pulmonary vascular resistance, and pulmonary endarterectomy.
BNP: B-type natriuretic peptide; CI: confidence interval; HR: hazard ratio; mPAP: mean pulmonary arterial pressure; PEA: pulmonary endarterectomy.
Hemodynamics of operated patients improved: post-surgical mPAP in this group was 27.7±14.3 mmHg, with a statistically significant decrease of 23.8 mmHg (95% CI: 11.8-35.8; p=0.002). Residual pulmonary hypertension was identified in three of the patients who survived the procedure (21.4%), requiring pulmonary vasodilator therapy during follow-up. WHO FC also improved in 92.9% of patients, with most patients in class I following PEA (64.3%).
DiscussionIn this paper, our data illustrated the symptomatic burden of CTEPH, reinforced the positive impact of PEA on hemodynamics, mortality and morbidity, and identified characteristics associated with a worse prognosis.
Although low awareness of CTEPH among physicians could have led to later referral of patients to our center, the baseline characteristics of our patients, namely age, gender, functional class, hemodynamic profile and 6MWT, were similar to previous large studies on CTEPH, suggesting the same disease progression status as in other centers.4,11,25–27
We found that PEA provided better prognosis and a numerical trend for lower mortality. This is not surprising considering the poor prognosis for inoperable CTEPH, which has three-year survival of 70% as documented in the international CTEPH registry.25 In comparison, three-year survival for patients who underwent PEA was 89%.25 Recently, Kallonen et al.28 compared survival after PEA to survival in the general population at 15 years. The authors found that although survival was shorter in the PEA group, the difference in life expectancy between the groups was small.
When perioperative death was excluded, the survival rate of patients undergoing PEA was higher than that of pharmacological treatment. This finding is not unexpected as most mortality is known to be immediately associated with the complex procedure. Surgical experience is a necessary requirement for better outcomes, as shown by the results of Korsholm et al.,29 as in-hospital mortality decreased from 22.6% in the first decade to 4.3% in the second decade of experience, and from the United Kingdom National Cohort,12 which showed significant improvement in 30-day mortality from the first half of the cohort to the latest (13.2 to 2.4%, respectively).
Regarding overall prognosis, we identified baseline serum BNP and mPAP as well as inoperability as independent predictors of worse outcomes. This finding is in line with previously published studies.15,30,31 Other commonly identified risk factors for poor prognosis, like cardiac index and distance on 6MWT,13,30 did not show an independent association with worse prognosis in our study, probably due to the small sample size.
Surgical treatment was refused by 20% of our patients. This emphasizes the difficulty of treating CTEPH without a national surgical center and the reluctance of the population to go abroad for a complex operating, resulting in only one third of patients undergoing PEA. While this figure is still below the numbers reported in larger studies,4,25 it shows an improvement since 2013, when only 15.2% of incident CTEPH patients in a nationwide registry underwent the procedure.32 Such increases have also recently been reported by other PH treatment centers in the country, with 29.2-50% of patients undergoing PEA,33–35 probably reflecting better access to the procedure. Previously, PEA was not routinely available in Portugal and patients were referred to foreign centers, but the initial experience of a Portuguese PEA center was recently published.24
In the last two decades BPA has emerged as a novel alternative for inoperable CTEPH.36,37 In our cohort only two patients underwent this procedure after being considered inoperable by the PEA center's multidisciplinary team. Considering the very promising results from the Japanese and French registries, establishing benefits in symptom relief, hemodynamics and survival in inoperable patients,22,38 and the recent availability of the procedure in Portugal,39,40 increased referral for BPA can be expected in the future.
Our study presents some limitations. The small sample size from a single center is the most obvious, and partially limits our analysis of outcomes. Other limitations are the fact that patients underwent PEA at two different centers with different levels of expertise; the low rate of operated patients; and the differences in baseline characteristics between the two groups. The changes in care and referral of CTEPH patients over the last decade may also affect the interpretation of our results and may limit the external validity of our sample.
ConclusionsCTEPH is a condition with a poor prognosis but is potentially curable. PEA provided a better overall prognosis than non-surgical therapy, improving symptoms and hemodynamics, with a numerical trend for lower mortality. Our results reinforce the importance of (1) referral centers for disease diagnosis and management and (2) prompt referral to PEA centers of patients that fit operability criteria. Baseline hemodynamics and serum BNP at diagnosis were associated with higher mortality in CTEPH patients.
FundingThis study was financed by national funds via FCT (Foundation for Science and Technology) through the Strategic Project UID/NEU/04539/2019, UIDB/04539/2020, UIDP/04539/2020 (CIBB) and FEDER (RIGHT-2H POCI-01-0145-FEDER-032414).
Conflicts of interestThe authors have no conflicts of interest to declare.