Idiopathic symptomatic non-sustained ventricular arrhythmias (INVA), including premature ventricular contractions and non-sustained ventricular tachycardias, are generally considered benign. Symptoms and left ventricular dysfunction are indications for treatment with antiarrhythmic drugs. The SCN5A gene encodes part of the voltage-dependent sodium channel type V alpha, which is the primary target of flecainide. The H558R polymorphism of this gene has been associated with a better response to flecainide in atrial fibrillation. The aim of this study is to describe the protocol of the CARFLECT IV clinical trial, which will compare the efficacy of flecainide and carvedilol in reducing INVAs and their consequences, and to assess a potential interaction with the H558R polymorphism.
MethodsA randomized, third party-blinded, crossover trial will be conducted in adults with frequent INVAs. Each treatment will be administered for 12 weeks. Thirty-two patients will be recruited. The primary objectives are to compare the reduction in arrhythmia burden before and after each treatment, as well as to perform a subsequent multivariate analysis that accounts for potential interactions with the H558R polymorphism. Multiple secondary objectives have been established: changes in quality of life, left ventricular ejection fraction, global longitudinal strain, and the occurrence of adverse events.
ResultsThe results of the CARFLECT IV trial are not yet available.
ConclusionsThis study will be the first clinical trial focused on adults with INVA. The information it provides may influence the choice of pharmacological therapy for the treatment of these arrhythmias.
As taquicardias ventriculares não sustentadas (TVNS) idiopáticas geralmente são consideradas benignas. Sintomas e disfunção ventricular esquerda são indicações para tratamento com fármacos antiarrítmicos. O gene SCN5A codifica parte do canal de sódio dependente de voltagem tipo V alfa, que é o principal alvo da flecainida. O polimorfismo H558R nesse gene tem sido associado a uma melhor resposta à flecainida na fibrilhação auricular. O objetivo deste estudo é apresentar o protocolo do ensaio clínico CARFLECT IV, que irá comparar a eficácia da flecainida e do carvedilol na redução das TVNS idiopáticas em doentes assintomáticos e de suas consequências clínicas, assim como investigar uma possível interação com o polimorfismo H558R.
MétodosSerá realizado um ensaio clínico randomizado, cego a terceiros e com crossover, em adultos com TVNS idiopáticas sintomáticas frequentes. Cada tratamento será administrado durante 12 semanas. Serão recrutados 32 participantes. O objetivo primário será comparar a redução da carga de arritmias antes e depois de cada tratamento e estimar potenciais interações com o polimorfismo H558R. Diversos objetivos secundários foram estabelecidos: alterações na qualidade de vida; avaliação ecocardiograáica da fração de ejeção do ventrículo esquerdo (FEVE) e STRAIN GLS; e a ocorrência de eventos adversos.
ResultadosOs resultados do ensaio CARFLECT IV ainda não estão disponíveis.
ConclusõesEste estudo será o primeiro ensaio clínico focado em adultos com TVNS idiopáticas sintomáticas. Os resultados decorrentes do estudo poderão vir a influenciar a escolha da terapêutica farmacológica, de modo que esta seja dirigida a essas arritmias.
Idiopathic non-sustained ventricular arrhythmias (INVAs) are those that occur in patients without structural heart disease. They include premature ventricular contractions (PVCs) and non-sustained ventricular tachycardias. These arrhythmias are generally considered to follow a benign clinical course2; however, in some patients, they may cause symptoms and/or impair left ventricular ejection fraction (LVEF).3,4
The indication to treat these arrhythmias is clearly established only when they impair LVEF and/or cause symptoms, and this treatment can be performed using either pharmacological therapy or interventional approaches.5 Reduction of the burden of PVCs has been shown to improve symptoms, LVEF, and left ventricular GLS (global longitudinal strain).6–8
Multiple drugs have been tested for the treatment of this condition, with most of the evidence derived from observational studies involving a small number of patients.5,9 Beta-blockers, calcium channel blockers, and flecainide are the drugs of choice. Good results have also been achieved through catheter ablation targeting the origin of the arrhythmia.
According to the 2022 European guidelines on ventricular arrhythmias, ablation, beta-blockers, and non-dihydropyridine calcium channel blockers are considered first-line therapies, while flecainide has been relegated to a second-line option.5 This recommendation was made despite the lack of robust evidence supporting it, as no studies have demonstrated the superiority of beta-blockers or calcium channel blockers over flecainide.
Until 2024, there were no randomized studies comparing beta-blockers to flecainide; however, observational studies suggested that flecainide might be more effective.10,11
In 2024, an open-label, crossover clinical trial comparing flecainide and metoprolol was published, involving a sample of 19 pediatric patients. Flecainide demonstrated a greater reduction in the number of PVCs compared to metoprolol (−10.6% vs. −2.4%; p=0.031).12
It should be noted, however, that the response to flecainide shows significant interindividual variability. The H558R polymorphism in heterozygosity has been associated with a better response to flecainide in atrial fibrillation.1 This polymorphism belongs to the SCN5A gene, which encodes the 1.5 subunit of the voltage-dependent sodium channel type V alpha, the primary therapeutic target of flecainide.
ObjectivesDue to the lack of clinical trials identifying the most suitable antiarrhythmic for PVCs in adults12 and the absence of data on the interactions of the H558R polymorphism with the treatment of these arrhythmias, we decided to conduct this clinical trial. Our study will compare the efficacy of flecainide and carvedilol in the treatment of these arrhythmias, with the aim of providing insights into the most effective therapeutic approach.
It is important to note that when treating INVAs, the clinician's ultimate goal is not to reduce the number of ectopic beats, but to prevent their consequences, which is to improve symptoms and left ventricular function. For this reason, the secondary objective of this study will be the comparison of the effect of these drugs on left ventricular systolic function and quality of life.
Material and methodsChoice of drugsWe decided to compare flecainide and a beta-blocker as they are the two most used drugs in our setting for the treatment of INVA. Among beta-blockers, we chose carvedilol due to its dosing schedule being similar to that of flecainide and because studies suggest that carvedilol may be superior to other beta-blockers in preventing ventricular arrhythmias.13
Study design and participantsThe study “CARvedilol versus FLECainide Treatment in Idiopathic Ventricular Extrasystoles and Tachycardias (CARFLECT IV)” is an open-label, prospective, randomized 1:1, and single-center study conducted in a tertiary care hospital. Blinding is limited to third parties. Patients were screened from among all individuals followed at the only cardiology service of a healthcare area covering approximately 450000 people.
Inclusion and exclusion criteriaInclusion- •
Patients with INVA with a minimum of 1000 ventricular beats recorded on a 24-hour Holter, causing symptoms and/or a slight reduction in LVEF (40–55% in women and 40–50% in men).
- •
Adult patients capable of providing informed consent.
- •
Allergy or adverse effects after prior use of flecainide and/or carvedilol.
- •
Previous use of flecainide and/or beta-blockers at therapeutic doses for the same indication as in the study. Patients initiated on low doses without proper titration will not be excluded.
- •
Rhythm disturbances in patients without peacemaker: spontaneous bradycardia under 55 bpm. Second- or third-degree atrioventricular block.
- •
QRS complex duration >120 ms.
- •
Hepatic impairment.
- •
Glomerular filtration rate <30 ml/m2/min.
- •
First-degree atrioventricular block with PR >220 ms.
- •
Significant heart failure.
- •
History of structural heart disease, including ischemic heart disease, moderate or severe valvular heart disease, moderate or severe left ventricular hypertrophy, cardiac surgery, sarcoidosis, congenital heart disease (including Brugada syndrome, long QT syndrome, short QT syndrome, cardiomyopathy, and other less common conditions) and LVEF <40%.
- •
Presence of accessory pathways.
- •
Pregnancy and breastfeeding, as carvedilol is not well studied in this context and should be avoided if alternative options are available. Women of childbearing potential will undergo a pregnancy test (urine B-HCG) before starting treatment, and if not already on highly effective contraception, oral contraceptives will be prescribed during treatment. Highly effective contraceptive methods include tubal ligation, combined oral contraceptive pills, and intrauterine devices.
- •
Prior electrophysiological procedures will constitute an exclusion criterion only if they were performed to ablate malignant ventricular arrhythmias.
- •
One or more first-degree relatives with a diagnosis of Brugada syndrome.
All patients treated by the cardiologists participating in the study who meet all the inclusion criteria and none of the exclusion criteria will be consecutively included for participation.
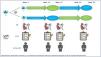
Patients will be randomized to receive either flecainide followed by carvedilol or carvedilol followed by flecainide. Patients will be followed for a total of three years and 26 weeks: 12 weeks with one drug, one week–two months of washout, and 12 weeks with the subsequent drug, plus an additional week to report adverse events occurring immediately after drug withdrawal. Follow-up will also include a telephone assessment at one year and another at three years (graphical abstract).
Between the washout period and the initiation of the second drug, an additional period of up to two months may be established to allow proper scheduling of subsequent visits. During this period, the patient may resume the drug already tested if deemed necessary by their clinician, but they must remain off treatment during the week prior to starting the second drug in the study.
Before initiating treatment, a baseline electrocardiogram, Holter ECG, standardized echocardiogram (ETT), and SF-36 quality of life questionnaire will be performed. During the drug titration phase, weekly in-person clinical evaluations will be conducted, including dose adjustments if indicated, along with Holters, ETTs, and ECGs. Once titration is completed, evaluations will be conducted every four weeks via telephone. After completing the 12-week follow-up for each drug, the same tests performed before starting the drug (ECG, baseline Holter ECG, standardized ETT, and SF-36 quality of life questionnaire) will be repeated. These results will be compared with those obtained before starting the drug. One week later, the patient will be contacted by phone to assess the occurrence of adverse events in the days following drug discontinuation.
Titration will be performed with the following doses:
- •
Oral flecainide: 50 mg every 12 hours, 100 mg every 12 hours, 150 mg every 12 hours (the highest dose will only be administered if GFR >60 ml/min and body weight >75 kg).
- •
Oral carvedilol: 6.25 mg every 12 hours, 12.5 mg every 12 hours, 25 mg every 12 hours.
The maximum required dose will be considered achieved when the burden of PVCs has been reduced by at least 85%.
If mild dose-dependent toxicity occurs during titration, the dose will be reduced to the last tolerated level. In the event of a severe dose-dependent or any non-dose-dependent adverse effect, treatment will be discontinued immediately. The following will be considered indicative of dose-dependent toxicity:
- •
In a 12-lead ECG: PR >250 ms and/or resting sinus bradycardia <50 bpm.
- •
In Holter monitoring: asymptomatic sinus pauses >3 seconds or atrioventricular block of Mobitz II, high-grade, or third-degree.
- •
For patients on flecainide, a QRS widening >25% will also be considered indicative of overdosing.
- •
Symptoms such as asthenia or general discomfort will be considered signs of overdosing. Digestive discomfort is also recognized as a potential adverse effect of flecainide.
Serious adverse events will be defined as those requiring hospital admission or resulting in the patient's death.
For patients receiving flecainide, low doses of atrioventricular node blockers (beta-blockers or calcium channel blockers) may be administered at the clinician's discretion to prevent class 1C-related flutter.
EndpointsPrimary endpoint- •
Difference between the two groups in the mean value of the ratio obtained by dividing the number of ventricular beats recorded on the 24-hour Holter ECG performed before the initiation of treatment by the number of ventricular beats recorded on the 24-hour Holter ECG performed after treatment completion.
- •
Multivariate analysis using logistic regression will include the treatment received and the presence of the H558R polymorphism in heterozygosity. This endpoint will be published later, as the analysis of these samples is part of the protocol for a broader observational study, and its evaluation may be delayed due to budgetary constraints. Patients will be allowed to participate in the clinical trial even if they decline genetic testing for the H558R polymorphism.
- •
Comparison of the absolute reduction in the number of ventricular beats recorded on the 24-hour Holter ECG before initiating each treatment versus after reaching the target doses. The analysis will be performed on an intention-to-treat basis.
- •
Comparison of the percentage of patients achieving a reduction of more than 80% in the absolute number of ventricular premature beats between the two groups.
- •
Subgroup/multivariate analysis for the primary endpoint based on the origin of the ventricular arrhythmia.
- •
Improvement in quality of life assessed using the SF-36 questionnaire.
- •
Changes in LVEF and left ventricular GLS between the baseline echocardiogram and the echocardiogram performed at the end of treatment with each drug.
- •
Number of patients experiencing any adverse event during follow-up with each drug.
- •
Number of patients with serious adverse events associated with each drug. Serious adverse events will be defined as those requiring hospital admission or resulting in the patient's death.
- •
Although these treatments are considered to have no effect on arrhythmias once discontinued, this has not been previously studied in an interventional manner. Our study will compare the number of ventricular beats recorded before each treatment with those recorded after the washout week, to confirm the absence of a long-term arrhythmia-suppressing effect. While such an effect has not been previously described and is unlikely given the drugs’ mechanisms of action, this will be systematically evaluated.
Additionally, two follow-ups will be conducted: one year after completing the treatment and another three years after completing the treatment. These follow-ups aim to evaluate the long-term treatment ultimately chosen by the patient's clinician, implemented freely and in a non-randomized manner.
H558RAll patients who agree to participate in the clinical trial will also be invited to take part in an observational study on the effects of the H558R polymorphism on the response to flecainide. Patients may decline genetic sampling without being excluded from the clinical trial. The presence of this polymorphism will be assessed using a blood sample obtained by venipuncture. The information from both studies may be combined if patients consent. For more information about the genetic study, refer to the supplementary material.
Other variablesIn addition to the variables included in the endpoints, anthropometric data such as height and weight, comorbidities, and concomitant treatments will be collected. Data on date of birth, date of diagnosis, and start date of each treatment will also be recorded. Heart rate, glomerular filtration rate, and toxic substance use will be documented, as well as electrocardiographic variables such as QRS complex duration.
Sample sizeAs we anticipate a 90% reduction in the number of PVCs in the flecainide group and a 75% reduction in the carvedilol group, with a standard deviation of 25%, an alpha error of 0.05, and a statistical power of 0.8, a sample size of 58 patients would be required. As a crossover design will be used, 29 patients will be sufficient. Assuming a 10% dropout rate due to loss to follow-up or adverse effects, a total of 32 patients will be needed. Patients will be recruited until the target sample size is achieved.
Statistical analysisFor the analysis of continuous quantitative dependent endpoints, linear regression, Student's t-test, and/or the Wilcoxon signed-rank test will be used, as appropriate. Normality will be assessed using the Shapiro–Wilk test (significant if p<0.1).
For the analysis of discrete quantitative dependent endpoints, Student's t-test, ANOVA, and logistic regression will be used, as appropriate.
For the analysis of qualitative dependent endpoints, logistic regression and chi-square testing will be used, as appropriate.
EthicsThe clinical trial has been registered with the European Medicines Agency under the code 2023-510362-26-00 and approved by the relevant authorities. Similarly, the H558R sub-study was approved by the ethics committee of our institution. All procedures will be conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent will be obtained from all participants prior to enrollment.
Current situationRecruitment has commenced, and the first three patients have already been enrolled in the study.
ResultsAlthough the study has already started, only three patients have completed the follow-up so far. No analysis will be performed until the follow-up of all patients is completed.
DiscussionIdiopathic symptomatic non-sustained ventricular arrhythmias are a common condition that can impair patients’ quality of life and entail significant healthcare costs. The CARFLECT IV study is the first randomized trial aimed at determining the best pharmacological treatment for adults with these arrhythmias. This issue has never been previously addressed through a clinical trial, leading to debates and changes in recommendations within European guidelines without reaching consensus.5 Observational studies and studies in children suggest that flecainide may be superior to beta-blockers,10,11 despite the 2022 European ventricular arrhythmia guidelines designating beta-blockers and calcium channel blockers as first-line drugs.5 The primary objective of this study is to compare the reduction in arrhythmia burden between the two groups. Given the short half-life of these drugs and the theoretical absence of residual effects following discontinuation, a crossover design with a washout period of at least one week was selected. Additionally, multiple secondary objectives have been included to assess changes in LVEF, symptomatic improvement, and the occurrence of adverse events.
Furthermore, to the best of our knowledge, this will be the first clinical trial to study the interaction of the H558R polymorphism with two antiarrhythmic drugs and the first study to examine the role of this polymorphism in the treatment of ventricular arrhythmias.
LimitationsThis study has several limitations, primarily due to the small target sample size (n=32), which may result in insufficient statistical power to detect moderate differences between groups or to assess low-incidence endpoints such as adverse events.
The use of a minimum of 1000 PVCs as an inclusion criterion is somewhat arbitrary. Furthermore, it does not take into account the percentage of PVCs, even though this is one of the study endpoints.
Additionally, the crossover design also presents challenges, mainly due to potential lifestyle changes during the study that could influence the effect size observed during the first treatment phase. For example, differences between pre- and post-treatment Holter results could reflect a combination of lifestyle changes and drug effects. Another potential limitation of a crossover approach could be spontaneous remissions. However, these should not pose a significant limitation, as they are typically reported after longer follow-ups, with a low spontaneous resolution rate (<15%) at six months.2
The requirement for women of childbearing age to initiate oral contraception to participate in the study could potentially influence arrhythmic burden. However, thanks to the crossover design and the fact that these patients will receive oral contraception during both treatment periods, the risk of bias is reduced.
An additional limitation of the crossover design is that, if the first treatment normalizes LVEF and GLS), it may be difficult to assess the effects of the second treatment on this parameter. This is because, if the second treatment were effective, no changes might be observed given that function was already normalized before its initiation; conversely, if it were ineffective, no changes might be seen either, as 12 weeks may not be sufficient for dysfunction to recur. This limitation could compromise the statistical power to detect differences in LVEF and GLS.
All these problems are partially addressed by the 1:1 randomization of patients to each treatment arm. Furthermore, the study is only blinded to third parties, which means that blinding will not be implemented for the patient or the treating physician. However, echocardiographic studies and Holter-ECGs performed at the beginning and end of each treatment period will be assessed in a blinded manner. In addition, the database will be coded so that the statistician remains unaware of treatment allocation. This approach minimizes the risk of bias in the primary outcome and in the secondary endpoints related to arrhythmic burden and imaging, although the possibility of bias in the assessment of quality of life cannot be excluded.
Finally, this is a low-intervention clinical trial designed to compare the real-world use of both treatment strategies. For this reason, we allowed the co-administration of low-dose beta-blockers with flecainide to prevent class 1C-related atrial flutter when considered appropriate by the treating physician. If, upon completion of recruitment and follow-up, a substantial number of patients in the flecainide arm are found to have received concomitant low-dose beta-blockers, this could lead to the conclusion that flecainide is superior to optimally dosed beta-blockers, when in fact what may have been demonstrated is that flecainide in combination with low-dose beta-blockers is superior. Nevertheless, this would also represent a clinically relevant finding. Therefore, we do not believe this limitation undermines the value of the study. In the end publication, we will report on the number of patients who received beta-blockers in combination with flecainide, and a subgroup analysis of these patients may also be performed.
ConclusionsIdiopathic symptomatic non-sustained ventricular arrhythmias are a highly prevalent condition; however, there is a lack of robust evidence to guide the selection of the most effective treatment.
Our trial is the first to focus on adult patients with INVA. Moreover, we not only evaluate the reduction in the number of ectopic beats but also assess changes in quality of life and echocardiographic parameters.
Finally, our study incorporates the investigation of the H558R polymorphism, which has been associated with greater efficacy and reduced toxicity of flecainide in patients with atrial fibrillation.
Thus, this is the first clinical trial in adults on a common condition, the first to measure the effects on quality of life and cardiac imaging, and the first to study the effects of the H558R polymorphism on flecainide when used to treat an arrhythmia other than atrial fibrillation. The results of this study could lead to a change in the current recommendations regarding the first-line treatment for this condition.
CRediT authorship contribution statementAll authors have contributed to the development and writing of this publication. The publication has been created based on the protocol previously developed by Mauro Trincado Ave, under the supervision of Moisés Rodríguez, with the collaboration of the other authors. All of them will play a role in the conduct of the clinical trial described herein.
Ethical approvalThis trial was approved by the European Medicines Agency and the local ethics committee. It is registered with the agency under Clinical Trial Registration Number (3/5/24): 2023-510362-26-00.
Consent to participateInformed consent will be obtained from all patients included in the study.
Declaration of generative artificial intelligence (AI) and AI-assisted technologies in the writing processDuring the preparation of this article, the author used ChatGPT in order to assist with English writing, as it is not the authors’ native language. After using this tool/service, the author reviewed and edited the content as needed and they take full responsibility for the content of the publication.
FundingThis study has been classified as a low-intervention study and will be funded with internal departmental resources.
Conflicts of interestNone declared.