Cangrelor is an intravenous adenosine triphosphate analog that reversibly blocks P2Y12 receptor-mediated platelet activation.1 It has a quick onset of action and a short plasma half-life, enabling complete platelet function recovery within 60 minutes of stopping the infusion.2,3 Cangrelor is approved for co-administration with aspirin to reduce thrombotic events in patients with coronary artery disease, undergoing percutaneous coronary intervention (PCI), who have not received an oral P2Y12 inhibitor (P2Y12i) prior to the procedure and in whom oral P2Y12is are not feasible.4
Stented patients who need urgent surgery are likely to experience an early suspension of dual antiplatelet therapy (DAPT), increasing the risk of stent thrombosis, or postponement of surgery due to bleeding risk concerns.1 An effective and safe antiplatelet bridging therapy is of particular relevance in this setting.
A 79-year-old man with rectal cancer under neoadjuvant chemotherapy suffered a non-ST-segment elevation myocardial infarction. Coronary angiography showed severe and complex osteal disease of the circumflex artery that was treated with a drug-eluting stent. The patient was considered to be at high thrombotic risk and DAPT with aspirin and ticagrelor was recommended for at least 12 months. Optimal timing for his rectal surgery was defined as 30 days after PCI. The high thrombotic risk associated with early suspension of P2Y12i was considered prohibitive. To avoid further delay in cancer surgery and worsening of the patient's prognosis, a special authorization for the off-label use of cangrelor as an antiplatelet bridging therapy was conceded by the Portuguese National Authority of Medicines and Health Products.
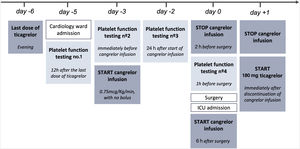
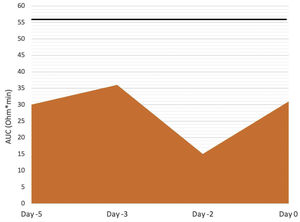
The patient was admitted to the cardiology ward 30 days after PCI for antiplatelet bridging therapy (Figure 1). Ticagrelor was stopped five days before surgery and cangrelor infusion was started 48 hours later (day -3), at a dose of 0.75 mcg/kg/min, without bolus. Cangrelor infusion was performed for three days and stopped two hours before surgery. Aspirin therapy was not suspended. We used the ROTEM®-Platelet platform (TEM International GmbH, Munich, Germany) to measure platelet function at four points, based on impedance aggregometry (IPA): Test 1, 12 hours after ticagrelor suspension (day -5); Test 2, immediately before starting cangrelor (day -3); Test 3, 24 hours after starting cangrelor (day -2); and Test 4, one hour before surgery (day 0). IPA results showed effective antiplatelet inhibition until surgery (Figure 2). The patient underwent a laparoscopic rectal resection, without acute bleeding complications. Six hours after surgery, cangrelor was restarted and subsequently stopped on the first morning post-surgery, when oral drug administration became possible. Ticagrelor was resumed, as a loading dose, immediately after cangrelor suspension. During the postoperative period, there were no bleeding or thrombotic complications.
ADP-TEM test results for evaluation of adenosine diphosphate receptor-mediated platelet aggregation. The repreented values are the area under the curve (AUC) of each test, a measure of the overall platelet aggregation. Reference values of AUC ADP-TEM for citrated samples are 56-139 Ω*min (the lower the value, the better the inhibition). AUC: area under the curve.
The use of cangrelor in this setting is supported by the BRIDGE trial,5 which showed a higher rate of platelet inhibition, without increasing major bleeding, in patients previously treated with oral P2Y12i awaiting cardiac surgery. Cangrelor bridging therapy should be started three days before surgery, maintained for two to seven days, and suspended one to six hours before surgery.1,2
Although glycoprotein (GP) IIb/IIIa inhibitors have also been used as a bridging therapy, their safety and efficacy in this setting have not been clearly established.6 They act in the final pathway of platelet aggregation, blocking the platelet response to all agonists.6 They present disadvantages compared to cangrelor. Their plasma half-life is longer and they should be stopped four to six hours before surgery.1,6 The ideal bridging dose has not yet been determined, so the recommendation is to use the same dose as in PCI, without a loading dose.1 Therapy should not exceed 72 hours to reduce bleeding risk.1
We report the first use of cangrelor as antiplatelet bridging therapy in Portugal. The absence of thrombotic or bleeding complications and the documentation of good platelet inhibition throughout bridging therapy suggest this strategy is safe and effective.
FundingThe authors have no funding
Conflicts of interestDr. Baptista reports receiving investigational grants from AstraZeneca.