We present the case of a patient with Takotsubo syndrome assessed by intracoronary flow and pressure guidewire, showing elevation of intracoronary pressures at the level of the anterior descending artery, and thus demonstrating a new therapeutic target in a still little understood etiopathogenic entity. The results of this test have never been previously reported in Takotsubo patients.
Apresentamos um caso dum paciente com síndrome de Takotsubo e a avaliação por fluxo intracoronário/fio de pressão, mostrando elevação das pressões intracoronárias ao nível da artéria descendente anterior, podendo demonstrar um novo alvo terapêutico numa entidade etiopatogénica ainda desconhecida. Os resultados deste teste nunca foram relatados previamente em pacientes de Takotsubo.
Takotsubo syndrome (TTS) is a cardiomyopathy that produces varying degrees of ventricular dysfunction, most often in the left ventricular apex, and is by definition reversible. Although the prognosis is generally good, in patients with comorbidities and worse prior functional class it is associated with more adverse events during follow-up.1
The pathophysiology of TTS is still largely unknown. Different hypotheses have been put forward, including cardiotoxicity from catecholaminergic hormone discharge, metabolic disturbance, impaired coronary microvascular circulation and epicardial coronary artery spasm.2 The most common clinical features are chest pain, elevated biomarkers of myocardial damage, and electrocardiographic alterations suggesting acute myocardial infarction (ST-segment elevation and deep T wave inversion) with transient left ventricular dysfunction in the absence of significant stenosis or plaque rupture in the coronary artery tree.3
The coronary microcirculation plays a crucial role in the development of TTS, including coronary pre-arterioles and arterioles (<500 μ in diameter) that modulate blood flow in response to neural, mechanical, and metabolic stimuli. A recent study assessed intracoronary flow in patients with TTS using the corrected TIMI frame count (cTFC) technique. It was observed that the cTFC in the anterior descending artery was significantly greater in TTS than in controls, the authors concluding that this could explain why the apex is often severely affected.4 However, a previous study found no significant differences in cTFC between 59 women with TTS and controls, which does not credibly support the theory of microvascular dysfunction as the single cause of TTS.5
Pressure wire measurements of fractional flow reserve (FFR) and index of microcirculatory resistance in TTS have been reported to demonstrate normal FFR but significant microcirculatory dysfunction,6,7 but there have been no published reports of the use of a pressure wire that simultaneously measures flow velocity in TTS.
The ComboWire XT® intracoronary guide (Volcano®, Philips) has a pressure transducer mounted proximal to the tip, enabling simultaneous pressure and flow velocity measurement when used with the ComboMap® system (Volcano, Philips). The parameters analyzed are:
- –
coronary FFR, defined as the ratio between mean pressure distal to the stenosis (Pd) and mean aortic pressure (Pa), a value <0.8 indicating a significant lesion;
- –
coronary flow reserve (CFR), defined as the ratio between coronary flow at maximal hyperemia and baseline conditions, a value >2 being considered normal;
- –
hyperemic stenosis resistance (HSR), defined as the ratio between the pressure gradient through the stenosis at maximal hyperemia (Pa-Pd) and mean peak velocity, normal values being defined as <0.8;
- –
hyperemic microvascular resistance (HMR), defined as the ratio between pressure in the distal part of the artery and mean peak velocity at that point; normal values are <2.8
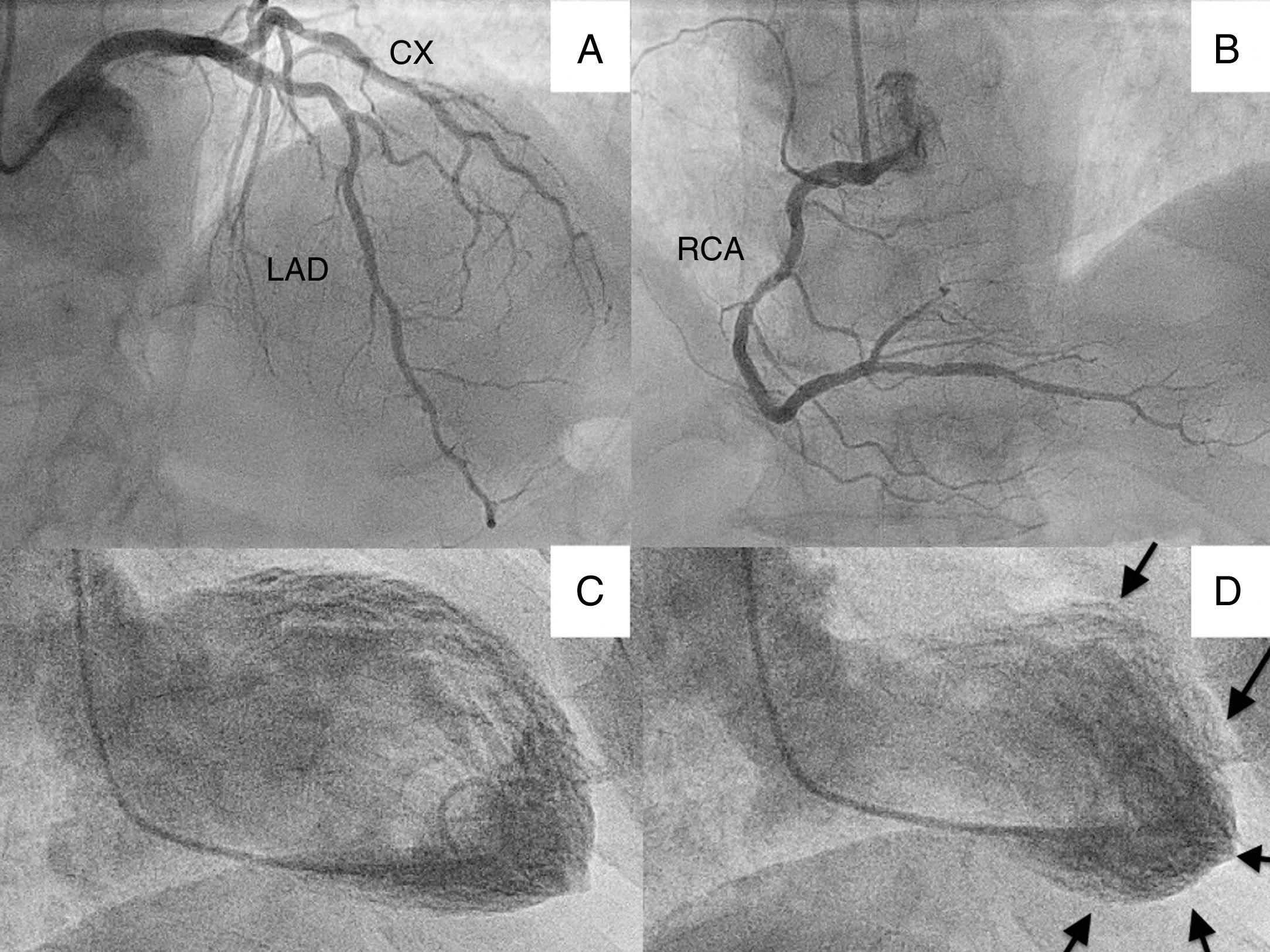
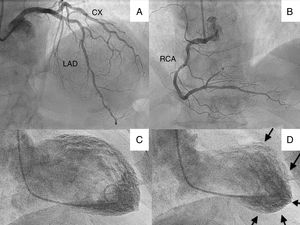
We present the case of a 70-year-old patient who went to the emergency department with chest pain and lateral ST elevation on the electrocardiogram. An emergent cardiac catheterization was performed, which showed epicardial arteries without significant angiographic lesions, and left ventriculography, which revealed extensive akinesia of the apex with mild systolic dysfunction (Figure 1).
(A) Cranial projection showing left anterior descending (LAD) and circumflex (CX) arteries without significant lesions; (B) cranial projection showing right coronary artery (RCA) without significant lesions; (C) ventriculography with left ventricle in end-diastole; (D) ventriculography showing left ventricle in end-systole, with apical akinesia (arrows).
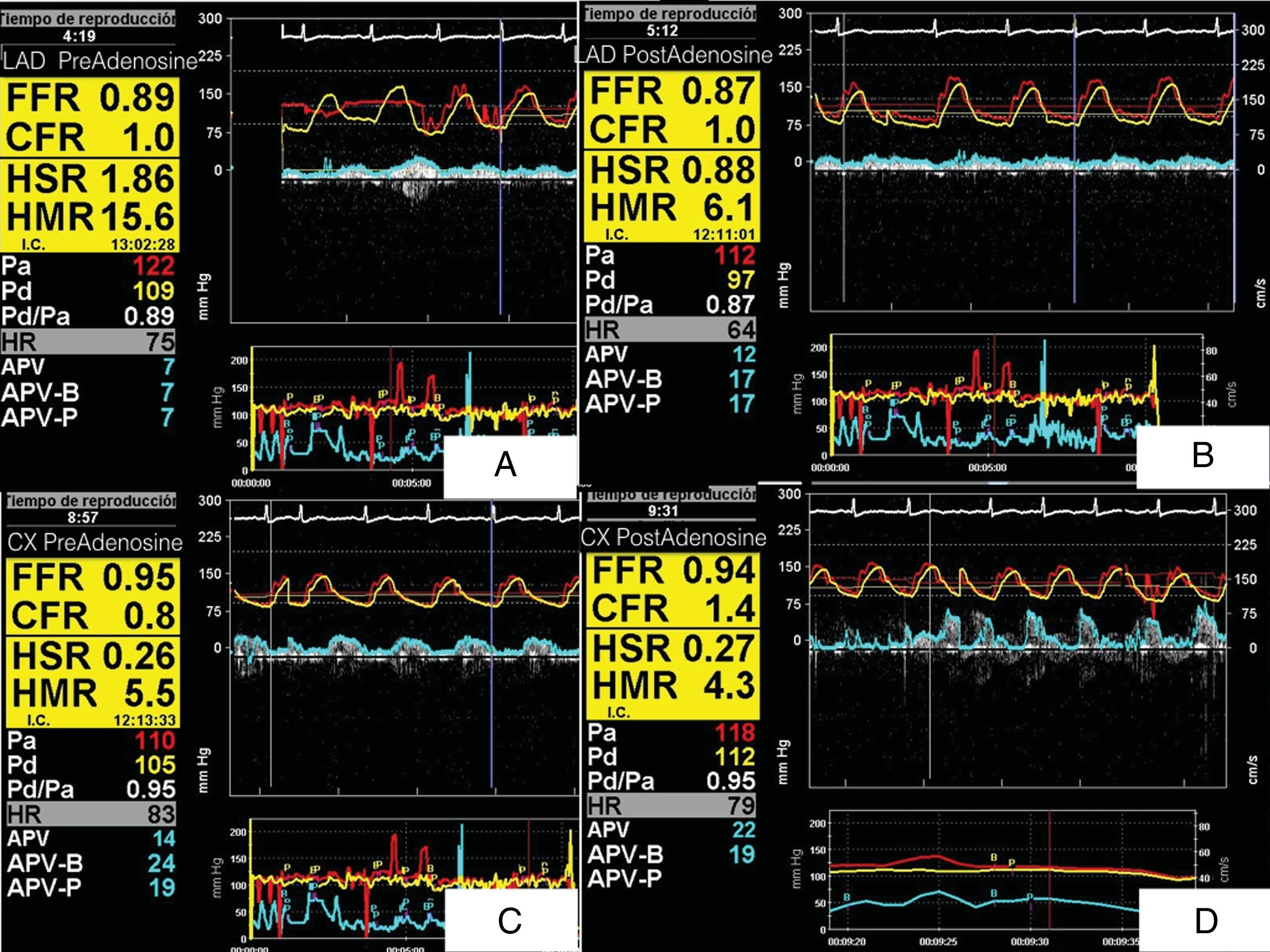
We decided to assess intracoronary pressure and flow using a ComboWire XT, at baseline and after intracoronary infusion of 300 pg of adenosine. This revealed FFR above 0.8 (not significant), low CFR (<2), normal HSR after adenosine infusion and high HMR (>2) in both left anterior descending (LAD) and circumflex arteries, strikingly high in the LAD (15.6), with a greater proportional response to adenosine (Figure 2). These findings may be partly due to the predominantly apical involvement.
(A) ComboMap® screenshot showing simultaneous pressure and flow measurements recorded in the distal left anterior descending artery (LAD) in baseline conditions, before adenosine administration (fractional flow reserve [FFR] 0.89, coronary flow reserve [CFR] 1, hyperemic stenosis resistance [HSR] 1.86, hyperemic microvascular resistance [HMR] 15.6); (B) following adenosine administration (FFR 0.87, CFR 1, HSR 0.88, HMR 6.1); (C) baseline values in circumflex artery before adenosine administration (FFR 0.95, CFR 0.8, HSR 0.26, HMR 5.5); (D) values in circumflex artery following adenosine administration (FFR 0.94, CFR 1.4, HSR 0.27, HMR 4.3). Note the high HMR before adenosine administration and the greater response to adenosine in the LAD.
The authors have no conflicts of interest to declare.


![(A) ComboMap® screenshot showing simultaneous pressure and flow measurements recorded in the distal left anterior descending artery (LAD) in baseline conditions, before adenosine administration (fractional flow reserve [FFR] 0.89, coronary flow reserve [CFR] 1, hyperemic stenosis resistance [HSR] 1.86, hyperemic microvascular resistance [HMR] 15.6); (B) following adenosine administration (FFR 0.87, CFR 1, HSR 0.88, HMR 6.1); (C) baseline values in circumflex artery before adenosine administration (FFR 0.95, CFR 0.8, HSR 0.26, HMR 5.5); (D) values in circumflex artery following adenosine administration (FFR 0.94, CFR 1.4, HSR 0.27, HMR 4.3). Note the high HMR before adenosine administration and the greater response to adenosine in the LAD. (A) ComboMap® screenshot showing simultaneous pressure and flow measurements recorded in the distal left anterior descending artery (LAD) in baseline conditions, before adenosine administration (fractional flow reserve [FFR] 0.89, coronary flow reserve [CFR] 1, hyperemic stenosis resistance [HSR] 1.86, hyperemic microvascular resistance [HMR] 15.6); (B) following adenosine administration (FFR 0.87, CFR 1, HSR 0.88, HMR 6.1); (C) baseline values in circumflex artery before adenosine administration (FFR 0.95, CFR 0.8, HSR 0.26, HMR 5.5); (D) values in circumflex artery following adenosine administration (FFR 0.94, CFR 1.4, HSR 0.27, HMR 4.3). Note the high HMR before adenosine administration and the greater response to adenosine in the LAD.](https://static.elsevier.es/multimedia/08702551/0000003800000011/v1_202002190705/S0870255120300044/v1_202002190705/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)


