Endothelial dysfunction and platelet activation have been highlighted as possible mediators in Takotsubo syndrome (TTS). Nevertheless, to date, evidence on the usefulness of antiplatelet therapy in TTS remains controversial. The aim of our study is to evaluate long-term prognosis in TTS patients treated with antiplatelet therapy (APT) at hospitalization discharge.
Material and methodsAn ambispective cohort study from the Spanish National Takotsubo Registry database was performed (June 2002 to March 2017). Patients were divided into two groups: those who received APT at hospital discharge (APT cohort) and those who did not (non-APT cohort). Primary endpoint was all-cause death. Secondary endpoints included the composite of recurrence or readmission and a composite of death, recurrence or readmission.
ResultsFrom a total of 741 patients, 728 patients were alive at discharge. Follow-up was performed in 544 patients, who were included in the final analysis: 321 patients (59.0%) in the APT cohort and 223 patients (41.0%) in the non-APT cohort. The APT cohort had a better clinical presentation and received more heart failure and acute coronary syndrome-like therapies (angiotensin converting enzyme inhibitors/angiotensin receptor blockers: 75.1% vs. 51.1%; p<0.001, betablockers: 71.3% vs. 50.7%; p<0.001, statins: 67.9% vs. 33.2%; p<0.001). After adjusting for confounder factors, APT at discharge was a protective factor for all-cause death (adjusted hazard ratio (HR) 0.315, 95% confidence interval (CI): 0.106-0.943; p=0.039) and the composite endpoint of all-cause death, recurrence or readmission (adjusted HR 0.318, 95% CI: 0.164-0.619; p=0.001) at month 25 of follow-up.
ConclusionPatients with TTS receiving APT at discharge presented better prognosis up to two-years of follow-up compared with their counterparts not receiving APT.
A disfunção endotelial e a ativação plaquetária são possíveis mediadores na síndrome de Takotsubo (STT). Até ao momento, a utilidade da terapia antiplaquetária no STT é controversa. O nosso objetivo é avaliar o prognóstico a longo prazo em pacientes com STT tratados com tratamento antiplaquetário (TAP) na alta da internação.
Material e métodosFoi realizado um estudo de coorte ambidirecional do banco de dados do Registro Nacional de Takotsubo da Espanha (junho-2002 a março-2017). Os pacientes foram divididos em aqueles que receberam TAP na alta hospitalar (cohorte-TAP) e aqueles que não receberam (cohorte não-TAP). O endpoint primário foi a morte global. Os endpoints secundários incluíram um composto de recorrência ou readmissão e um composto de morte, recorrência ou readmissão.
ResultadosDe 741 pacientes, 728 estavam vivos na alta. O acompanhamento foi realizado em 544 pacientes, que foram incluídos na análise final: 321 (59,0%) na cohorte-TAP e 223 (41%) na cohorte não TAP. A cohorte TAP mostrou melhor apresentação clínica e recebeu mais tratamentos de insuficiência cardíaca e (IECA/ARB: 75,1% versus 51,1%; p<0,001, betabloqueadores: 71,3% versus. 50,7%; p<0,001, estatinas: 67,9% versus 33,2%; p<0,001). Após o ajuste para fatores de confundimento, o TAP na alta foi um fator de proteção para a morte global (HR ajustado 0,315, IC95%: 0,106-0,943; p=0,039) e o composto de morte global, recorrência ou readmissão (HR ajustado 0,318, IC95%: 0,164-0, 619; p=0,001) até aos 25 meses.
ConclusãoPacientes com STT recebendo TAP na alta apresentaram melhor prognóstico até aos dois anos de acompanhamento em comparação com seus homólogos que não receberam TAP.
Takotsubo syndrome (TTS) is characterized by transient apical ballooning of the left ventricle and usually presents with symptoms and electrocardiographic signs mimicking an acute coronary syndrome (ACS).1,2 TTS was thought to be a benign and self-limiting entity. However, serious complications have been reported, with an incidence of thrombotic events or death during index hospitalization and follow-up comparable to ACS.3,4
The pathogenesis of TTS is not completely understood. High levels of catecholamines and a specific beta-adrenergic receptor distribution in the myocardium seem to support a causative link.5–8 Furthermore, catecholamines stimulate platelet activation,9–12 inflammatory activity and endothelial dysfunction, all of which have been observed in small observational studies in TTS13–15 and might be involved in complications. Also, antiplatelet therapy (APT) was initially described as an independent predictor for low incidence of major cardiovascular events during hospitalization for TTS16 and good prognosis in the short-term.17 For these reasons, in a non-negligible proportion of centers, it is common to administer APT in TTS during hospitalization and at discharge.
Recently, a meta-analysis18 of observational studies17,19–21 observed that antiplatelet therapy (APT) in the long-term in TTS is related with poor outcomes. However, the controversy continues because the strength of the meta-analysis conclusions is limited because they are mainly driven by the research of D’Ascenzo et al.21
The aim of our study was to analyze the impact of APT at discharge on long-term prognosis after an index hospitalization due to TTS based on the Spanish national registry.
Material and methodsStudy populationWe conducted an ambispective cohort study reviewing patients with a definite diagnosis of TTS included in the Spanish National Takotsubo Registry (RETAKO Registry) between June 2002 and March 2017. Patients were included prospectively from 2012 onwards, and were allowed to be included retrospectively. The first patient included was discharged in June 2002 and the last in March 2017. Inclusion criteria and methodology are described in depth in the original manuscript.3
Briefly, inclusion criteria were as per Modified Mayo Criteria22: (1) akinesia or dyskinesia of the apical and/or midventricular segments of the left ventricle with regional wall motion abnormalities that extended beyond the distribution of a single epicardial vessel; (2) absence of angiographic obstructive coronary artery disease, which was defined as a luminal obstruction of more than 50%; (3) new electrocardiographic abnormalities together with cardiac troponin elevation; (4) absence of pheochromocytoma or myocarditis; and (5) complete recovery of left ventricular function during follow-up.
The study protocol recommended an electrocardiogram at admission, at 24 and 48 hours; an early echocardiogram; and blood tests, including initial and peak troponin. Study protocol also included a three-month clinical and echocardiogram follow-up and at least a 12-month clinical follow-up. Follow-up data were allowed to be continued past the 12-month mark. All other clinical decisions, including treatment and timing of tests and discharge were left to the discretion of the treating physician.
Data were collected in a dedicated online data input application and included: baseline characteristics; past medical history; relevant blood tests; electrocardiographic, echocardiographic and angiographic findings, and medical treatment before, during and after hospitalization.
The protocol was submitted and received approval of the Medical Ethics Committee at the Hospital Clínico San Carlos in Madrid, Spain. The study was conducted in compliance with the Declaration of Helsinki and applicable local requirements. A written informed consent was obtained prior to the inclusion in the database.
Definitions and study endpointsThe objective of this study was to determine the prognostic impact of APT at discharge at 12-month mandatory follow-up and beyond. The null hypothesis was that being discharged on APT was not related to survival free of the primary endpoint of death; or survival free of the secondary combined endpoint of recurrence or readmission to a cardiology ward; or survival free of the secondary combined endpoint of death, recurrence or readmission.
Antiplatelet therapy status at discharge (APT cohort) was defined as a patient treated with aspirin and/or any P2Y12 inhibitor at discharge for an index hospitalization for TTS. Patient not receiving APT at discharge (non-APT cohort) was defined as a patient reported as not being prescribed aspirin or any P2Y12 inhibitor at discharge. All-cause death during follow-up was defined as the event of death being reported during the follow-up period. Recurrence of TTS was defined as the reappearance of similar symptoms reported during the follow-up period3. Readmission was defined as a readmission in a cardiology ward reported during the follow-up period.3 At least one echocardiogram record after discharge was deemed necessary to achieve survival status, even if further follow-up was not available.
Chronic kidney disease was defined as baseline serum creatinine >1.5 mg/dL.3 Mitral regurgitation was considered greater than mild when graded II-IV.3 Based on prior literature, advanced age was defined as an age >75 years for the multivariate analysis. Other continuous variables were transformed in dichotomous variables by using the third quartile of the distribution as a cut-off point.
Statistical analysisStatistical analyses were performed using SPSS Statistics 20.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables were explored for normal distribution with the Kolmogorov-Smirnov test. Variables following normal distribution were expressed as mean (standard deviation) and non-normally distributed variables were expressed as median (inter-quartile range (IQR)). Continuous variables were also explored using box plots to identify extreme values leading to data entry errors. Categorical variables were expressed as count (percentage). Comparisons between continuous variables were performed with t-student or U-Mann-Whitney tests as appropriate. Comparisons between categorical variables were performed with the chi-square or Fisher's exact tests as appropriate. All p values were two-tailed, with statistical significance set at a level of <0.05. Cases with missing values were excluded from analyses.
To evaluate the association between the exposition variable (APT at discharge) and the primary and secondary endpoints, we conducted a multivariate Cox survival regression model. Also, the results were plotted using Kaplan-Meier survival curves and compared with the log-rank test. To avoid biases derived from unequal follow-up in comparison groups, we tested the proportional hazards assumption with Kaplan-Meier survival curves and Cox regression models using time dependent covariables for the exposition variable. If hazards were not proportional over time for the exposition variable, we then identified the landmark in time in which the change in hazards occurred and expressed the hazard up to this point. In the first step and in order to adjust for possible confounders, exploratory univariate analyses, evaluating all variables in the study, were performed using the log-rank test. Variables with p values <0.05 were included in a backward stepwise Cox survival regression model. Results were reported as hazard ratios (HR), together with the 95% confidence intervals (95% CI) and p values.
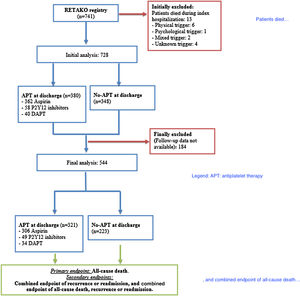
ResultsStudy flow-chart is depicted in Figure 1. From 741 patients included in the registry, 728 patients were alive at discharge and 380 patients were on APT and 348 patients were not on APT. Data for patients alive at discharge are shown in Supplementary material (Tables 1B, 2B, 3B and 4B). Follow-up was available in 544 patients who were included in the final analysis, 321 patients (59.0%) in the APT cohort and 223 patients (41.0%) in the non-APT cohort. Median follow-up for the overall cohort was 10.6 months (IQR 41.2); 13.2 months (IQR 51.4) in the APT cohort and 7.2 months (IQR 18.8) in the non-APT cohort.
Table 1 shows no differences in demographic and clinical baseline characteristics between groups. Table 2 shows clinical presentation characteristics, highlighting that the APT cohort presented a better clinical profile and shorter hospitalization compared with non-APT cohort. Significant blood test results, electrocardiographic, angiographic and echocardiographic data are expressed in Table 3. There were no differences in blood test results between study groups. Atrial fibrillation was less common in the APT cohort and the APT cohort had higher left ventricle ejection fraction at presentation and at recovery compared with the non-APT cohort. However, the presence of more than mild mitral regurgitation was more frequent in the APT cohort. Table 4 shows treatment before admission and at discharge. Before admission, any antiplatelet treatment, angiotensin convertase enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) and statins were more common in the APT cohort. Conversely, anticoagulants were less common in the APT cohort as compared to non-APT cohort. At discharge, ACEI or ARB, betablockers and statins were all more commonly prescribed in the APT cohort. Treatment with long-term anticoagulants was less common in the APT cohort.
Demographic data and baseline characteristics of patients with follow-up available.
| All patients with follow up available (n=544) | APT at discharge (n=321) | No APT at discharge (n=223) | p value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 72 (18) | 72 (16) | 71.5 (19) | 0.441 |
| Women, n (%) | 484 (88.6%) | 286 (88.8%) | 198 (88.4%) | 0.887 |
| Hypertension, n (%) | 359 (66.2%) | 213 (66.4%) | 146 (66.1%) | 0.994 |
| Hypercholesterolemia, n (%) | 308 (57.2%) | 185 (58.0%) | 123 (56.2%) | 0.673 |
| Diabetes mellitus, n (%) | 86 (16.1%) | 52 (16.5%) | 34 (15.6%) | 0.791 |
| Active smoking, n (%) | 77 (14.2%) | 51 (15.9%) | 26 (11.8%) | 0.172 |
| Obesity, n (%) | 86 (16.5%) | 53 (17%) | 33 (15.7%) | 0.701 |
| Chronic kidney disease, n (%) | 26 (4.8%) | 15 (4.7%) | 11 (5%) | 0.852 |
| COPD/asthma, n (%) | 80 (14.8%) | 45 (14.1%) | 35 (15.9%) | 0.553 |
| Ischemic heart disease, n (%) | 7 (1.3%) | 6 (1.9%) | 1 (0.4%) | 0.249 |
| Heart failure, n (%) | 7 (1.3%) | 2 (0.6%) | 5 (2.2%) | 0.129 |
| TIA/stroke, n (%) | 25 (4.6%) | 16 (5.0%) | 9 (4%) | 0.601 |
| NYHA class> I prior to hospitalization, n (%) | 106 (19.8%) | 66 (20.8%) | 40 (18.3%) | 0.481 |
APT: antiplatelet therapy; COPD: chronic pulmonary obstructive disease; NYHA: New York Health Association; TIA: transient ischemic attack.
Clinical presentation in patients with follow-up available.
| All patients with follow up available (n=544) | APT at discharge (n=321) | No APT at discharge (n=223) | p value | |
|---|---|---|---|---|
| Days of hospitalization, mean (SD) | 9.14 (8.25) | 7.8 (4.5) | 11.7 (12.2) | 0.039 |
| Trigger, n (%) | ||||
| Psychological | 212 (39.5%) | 128 (40.3%) | 84 (38.4%) | 0.658 |
| Physical | 124 (23.1%) | 65 (20.4%) | 59 (26.9%) | 0.079 |
| Mixed | 19 (3.5%) | 7 (2.2%) | 12 (5.5%) | 0.043 |
| None | 182 (33.9%) | 118 (37.1%) | 64 (29.2%) | 0.058 |
| Chest pain at presentation, n (%) | 0.002 | |||
| Angina at rest | 298 (55.5%) | 175 (54.9%) | 123 (56.4%) | 0.765 |
| Angina on exertion | 76 (14.2%) | 56 (17.6%) | 20 (9.2%) | 0.005 |
| Mixed | 3 (0.6%) | 2 (0.6%) | 1 (0.5%) | 0.680 |
| Atypical | 51 (9.5%) | 36 (11.3%) | 15 (6.9%) | 0.084 |
| None | 109 (20.3%) | 50 (15.7%) | 59 (27.1%) | 0.001 |
| Heart rate at presentation, (beats/min) median (IQR) | 85 (25) | 82 (22) | 90 (34) | 0.072 |
| Shock at presentation, n (%) | 41 (7.7%) | 15 (4.7%) | 26 (12.1%) | 0.002 |
| Worst Killip class during hospitalization >I, n (%) | 188 (35.2%) | 90 (28.4%) | 98 (45.2%) | <0.001 |
| Intraventricular thrombus, stroke or systemic embolism, n (%) | 25 (4.7%) | 14 (4.4%) | 11 (5.1%) | 0.835 |
| Pulmonary embolism, n (%) | 1 (0.2%) | 0 (0%) | 1 (0.5%) | 0.405 |
| Any bleeding, n (%) | 16 (3%) | 9 (2.8%) | 7 (3.1%) | 0.802 |
| Acute kidney injury, n (%) | 54 (10.2%) | 24 (7.7%) | 30 (14.0%) | 0.020 |
| Infection, n (%) | 121 (22.9%) | 71 (22.5%) | 50 (23.5%) | 0.802 |
| Non-invasive mechanical ventilation, n (%) | 36 (6.6%) | 21 (6.5%) | 15 (6.7%) | 0.932 |
| Mechanical ventilation, n (%) | 33 (6.1%) | 9 (2.8%) | 24 (10.8%) | <0.001 |
APT: antiplatelet therapy.
Significant blood tests, electrocardiogram, angiography and echocardiography in patients with follow-up available.
| All patients with follow up available (n=544) | APT at discharge (n=321) | No APT at discharge (n=223) | p value | |
|---|---|---|---|---|
| Hemoglobin (g/dL), median (IQR) | 13.3 (1.6) | 13.2 (1.7) | 13.4 (1.5) | 0.113 |
| Creatinine highest value (mg/dL), median (IQR) | 0.9 (0.36) | 0.89 (0.31) | 0.94 (0.43) | 0.255 |
| CK highest value U/L, median (IQR) | 190 (199) | 196 (175) | 178 (266) | 0.475 |
| Heart rhythm, n (%) | 0.064 | |||
| Sinus | 469 (88.1%) | 287 (90.5%) | 181 (84.6%) | 0.037 |
| Atrial fibrillation | 45 (8.5%) | 20 (6.3%) | 25 (11.7%) | 0.029 |
| Pacemaker | 10 (1.9%) | 4 (1.3%) | 6 (2.8%) | 0.199 |
| Other | 8 (1.5%) | 6 (1.9%) | 2 (0.9%) | 0.374 |
| ST elevation at presentation, n (%) | 320 (60%) | 198 (62.3%) | 122 (56.7%) | 0.202 |
| Right dominance, n (%) | 461 (88.5%) | 275 (89.3%) | 186 (87.3%) | 0.489 |
| LVEF (%) at diagnosis, mean (SD) | 43.3 (12.0) | 44.4 (12.0) | 41.3 (11.0) | 0.001 |
| LVEF (%) at recovery, mean (SD) | 62.9 (7.0) | 63.2 (9.5) | 62.4 (5.9) | 0.009 |
| More than mild MR at recovery (≥II/IV), n (%) | 135 (24.8%) | 95 (29.6%) | 40 (17.9%) | 0.002 |
APT: antiplatelet therapy; CK: creatine kinase; LVEF: left ventricle ejection fraction; MR: mitral regurgitation; SD: standard deviation.
Treatment prior to hospitalization and at discharge in patients with follow-up available.
| All patients with follow up available (n=544) | APT at discharge (n=321) | No APT at discharge (n=223) | p value | |
|---|---|---|---|---|
| Treatment previous to hospitalization | ||||
| Any antiplatelet treatment | 89 (16.4%) | 77 (24%) | 12 (5.4%) | <0.001 |
| ACEI/ARB, n (%) | 228 (41.9%) | 140 (43.6%) | 88 (39.5%) | 0.334 |
| BB, n (%) | 49 (8.9%) | 30 (9.3%) | 19 (8.5%) | 0.741 |
| Statins, n (%) | 142 (26.1%) | 90 (28.0%) | 52 (22.3%) | 0.218 |
| Anticoagulants, n (%) | 52 (9.6%) | 16 (5.0%) | 26 (16.1%) | <0.001 |
| Treatment at discharge | ||||
| ACEI/ARB, n (%) | 355 (65.3%) | 241 (75.1%) | 114 (51.1%) | <0.001 |
| Betablockers, n (%) | 342 (62.9%) | 229 (71.3%) | 113 (50.7%) | <0.001 |
| Diuretics, n (%) | 134 (24.6%) | 78 (24.3%) | 56 (25.1%) | 0.829 |
| Statins, n (%) | 292 (53.7%) | 218 (67.9%) | 74 (33.2%) | <0.001 |
| Anticoagulation, n (%) | 102 (18.8%) | 37 (11.5%) | 65 (29.1%) | <0.001 |
ACEI: angiotensin convertase enzyme inhibitor; APT: antiplatlet therapy; ARB: angiotensin receptor blocker; BB: betablocker.
As seen in Figure 1, follow-up data were not available in 184 patients. Fifty-four deaths were documented with a mortality rate of 6.43 deaths per 100 patient-years. Moreover, 74 events for the secondary endpoint of recurrence or readmission; and 116 events for the combined event of death, recurrence or readmission were reported. The 25-month mark was identified as the point in time at which hazards stopped being proportional and survival analyses were performed until this point.
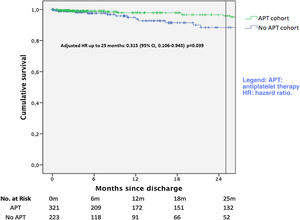
Survival free of the primary endpoint of all-cause death is plotted in Figure 2. There was a significant unadjusted protective effect of APT up to 25 months (unadjusted HR 0.325; 95% CI 0.146-0.880, p=0.025). After multivariate adjustment, APT remained as a protective factor (adjusted HR 0.315; 95% CI 0.106-0.943, p=0.039).
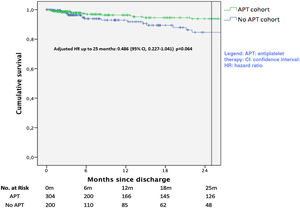
Survival analysis for the combined endpoint of recurrence or readmission is plotted in Figure 3. A significant unadjusted protective effect of APT up to 25 months was observed (unadjusted HR 0.439; 95% CI 0.211-0.915, p=0.028). After multivariate adjustment, this protective effect was found not to be statistically significant (HR 0.486; 95%CI 0.227-1.041, p=0.064).
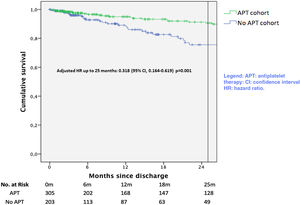
Survival analysis for the secondary combined endpoint of death, recurrence or readmission is plotted in Figure 4. There was a significant unadjusted protective effect of APT up to 25 months (unadjusted HR 0.39; 95% CI 0.215-0.705, p=0.002). After multivariate adjustment, APT remained as a protective factor (adjusted HR 0.318; 95% CI 0.164-0.619, p=0.001).
Multivariate analyses for independent predictors for primary and secondary endpoints are described in Supplementary material (Supplementary Tables 5B, 6B and 7B).
DiscussionThe main finding of our study is that APT prescribed at discharge, even after correcting for all possible confounders, appears to be related to a better prognosis in the first 25 months of follow-up in terms of all-cause death and death, recurrence or readmission.
Rationale for the use of antiplatelet treatment in the long-term in Takotsubo syndromeAlthough TTS pathophysiology is incompletely understood, mechanisms involved in TTS are multiple. In most cases psychological or physical stress trigger the cascade of events in TTS.3,4 It has been observed that an exaggerated response of the sympathetic system to stress generates an intense catecholamine drive that is related to myocardial stunning and an increment in platelet activation.5–8 Also, the elevation of catecholamine levels is associated with a worsening of endothelial function, showing an increment in endothelial dysfunction in TTS patients as compared to a matched control group.14
Some studies have shown that catecholamine stimulation causes increased platelet activation and up-regulation of fibrinogen receptors which is an essential step in platelet aggregation,9,12 and it has been demonstrated that patients admitted for a TTS acute event presented higher blood levels of catecholamines compared to patients presenting with an ACS.7,15 Hoever, this platelet activation may not be exclusively limited to the acute phase. In one observational study exploring platelet function during the acute phase and at follow-up, Nuñez-Gil et al. demonstrated that TTS patients, who had higher levels of adrenaline in the acute phase, displayed increased platelet reactivity at three months.15 In addition, these biological findings (platelet reactivity and endothelial dysfunction) could have a clinical translation, as highlighted by the fact that several studies have shown that the incidence of thrombotic events during follow-up in TTS were comparable to ACS.3,4 Therefore, the prolongation of APT with aspirin or PY212 inhibitors could be a therapeutic option in TTS long-term management. Also, this is reasonable since the clinical profile of these patients points to an important atherosclerotic risk.
Impact of antiplatelet treatment on Takotsubo syndrome long-term prognosisTo date, the evidence supporting long-term treatment for TTS is poor due to the lack of randomized clinical trials, and only treatment with ACEI or ARB has been associated with a reduction in TTS recurrences in a meta-analysis of observational studies.23 This means that there is no consensus regarding long-term management in this population.24
Despite pathophysiological data relating platelet activation to events in TTS, no study has demonstrated clinical benefit in extending APT in the long-term. A recent meta-analysis,18 including observational studies,17,19–21 observed that APT in the long-term increases all-cause mortality and a composite outcome of all-cause death, TTS recurrence, stroke/transient ischemic attack or myocardial infarction/coronary artery disease progression. Nevertheless, the conclusions of this meta-analysis should be interpreted with caution. First of all, the study conducted by D’Ascenzo et al.,21 which accounts for more than 90% of the weight for overall mortality and for the combined endpoint, could have conditioned the results. Likewise, it is striking that, although statistical significance was not reached in the meta-analysis, the probability stroke/transient ischemic attack or myocardial infarction/coronary artery disease progression was higher in the group of patients treated with APT, taking into account that these patients present a high proportion of cardiovascular risk factors and an elevated cardiovascular risk.
In our research, the vast majority of patients received simple APT with aspirin and only a minority of patients received P2Y12 inhibitors or dual APT, showing reduction in mortality, recurrence or readmission. In line with previous data reported by Dias,16 showing a reduction in major cardiovascular adverse events during hospitalization, our results would support lengthening antiplatelet therapy up to 25 months, mainly with aspirin. It is noteworthy that, in contrast to the work of D’Ascenzo et al.,21 in which the follow-up reached five years, our data show that the optimal prolongation for APT could be around two years. This leads us to hypothesize that there could be a temporary period after the presentation of a TTS in which APT could offer the greatest benefits, minimizing the risks.
LimitationsFirstly, this study is an observational study with inherent biases. Our data refer to the population of Spain and therefore should be considered carefully before being extrapolated to other geographical areas. Secondly, the clinical criteria for prescribing APT at discharge are unknown. Patients prescribed APT at discharge also received other heart failure-like medications, which might indicate that some clinicians preferred treating TTS based on the evidence of ACS and heart failure. Thirdly, patients in the APT cohort had a better clinical profile than the non-APT cohort. However, after multivariate analysis, APT remained an independent factor for good prognosis. Fourthly, time to follow-up was uneven between cohorts and there was no data on adherence to APT. However, the highest proportion of patient losses occurred in the non-APT cohort, which could penalize and underestimate the beneficial effect of APT. Fifthly, bleeding and other APT-related complications were not specifically checked during follow-up. This might affect the net clinical benefit favoring the APT group.
ConclusionProlongation of APT after TTS presentation is supported by a solid pathophysiological rationale. Although recent studies would advise against its long-term administration, our observations would seem to demonstrate the usefulness of APT up to two years after discharge in the TTS population. Randomized controlled trials, which adequately assess mortality, recurrences, and ischemic and bleeding events, are required to determine the role of long-term APT in TTS.
Conflicts of interestThe authors have no conflicts of interest to declare.