Diabetes is a major determinant of ischemic events after percutaneous coronary intervention (PCI). In a nationwide prospective registry, antiplatelet and glucose-lowering treatment regimens, and two-year clinical outcomes were studied in unselected patients with type 2 diabetes undergoing coronary stent implantation. The current analysis describes the population's baseline characteristics and the prescription patterns of anti-thrombotic and glucose-lowering drugs.
MethodsBetween January and November 2021, 1000 patients were enrolled in 12 Portuguese hospitals. In addition to clinical and procedural-related variables, thrombotic (DAPT score) and bleeding risks (PRECISE DAPT) were estimated, and medication (including planned duration of dual antiplatelet therapy) were recorded.
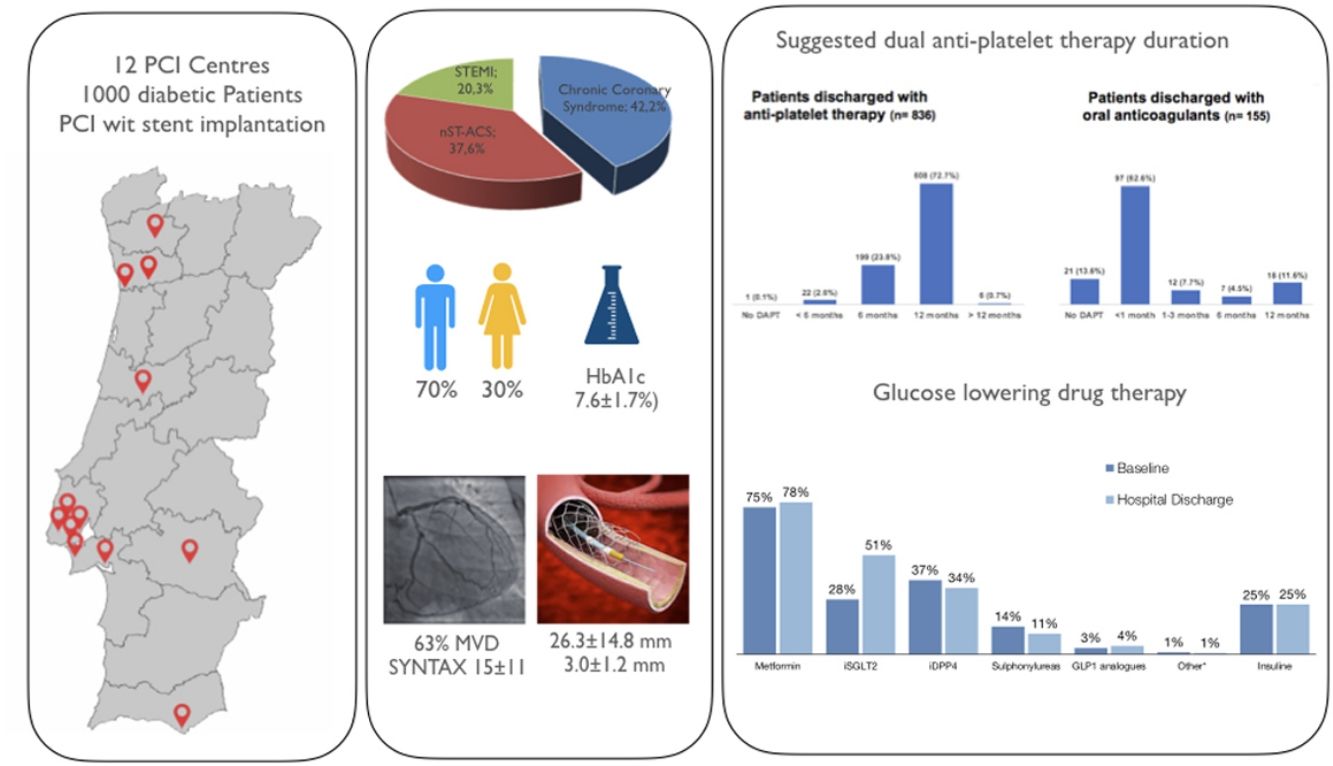
ResultsInclusion rate was relatively high (68.6%) among all eligible patients during the study period (mean age 68±10 years-old, and 70% of male gender). The indication for PCI was an acute coronary syndrome in 58% of cases and 63% had 2–3 vessel coronary artery disease (SYNTAX score 15.6±10.7; mean stent length and diameter 26.3±14.8 and 3.0±1.2 mm, respectively). Of patients not on oral anticoagulation, only 49.8% received potent P2Y12 inhibitors; overall recommendation for shorter DAPT regimens (<6 months,) was 26.5% and did not differ according to low vs. high bleeding risk (24.6% vs. 29.7%; p<0.125). In those also under anticoagulation, 62.6% received a recommendation for <30-day regimens, or no DAPT at all (13.6%). Prolonged DAPT (>12 months) was planned at baseline in 0.7% of the whole cohort and 1.2% of ACS patients. PCI complexity (but not CAD extent) was associated with DAPT duration. Self-reported duration of diabetes was >6 years in 57% (HbA1c 7.6±1.7%) and 12% had known microangiopathy at inclusion. SGLT2 inhibitors (28%) and GLP-1 analogues (3%) were used seldom at admission.
ConclusionsStandard six-to-12-month antiplatelet regimens were the most widely used, largely with acetylsalicylic acid and clopidogrel. DAPT duration was mostly related to PCI complexity and oral-anticoagulation. Metabolic control was off-target and guideline-directed treatment for diabetes was underused at admission (clinicaltrials.gov NCT04481997).
A diabetes é um dos principais determinantes do risco isquémico após intervenção coronária percutânea (ICP). Os regines de terapêutica anti-plaquetar e hipoglicemiante em doentes diabéticos submetidos a angioplastia com implantação de stent, assim como o prognóstico aos 2 anos, foram estudados num registo prospetivo multicêntrico de âmbito nacional. Presente análise reporta os resultados referentes às características basais dos doentes incluídos e aos padrões de prescrição.
MétodosEntre janeiro e novembro de 2021 foram recrutados 1000 doentes em doze hospitais portugueses. Para além das variáveis clínicas, angiográficas e relacionadas com o procedimento, foram especificamente estimados os riscos trombótico e hemorrágico, através dos cálculos dos scores DAPT e PRECISE-DAPT, respetivamente, e registadas as recomendações respeitantes à terapêutica farmacológica, incluindo o esquema proposto de dupla anti-agregação e a sua duração planeada.
ResultadosConsiderando toda a população elegível durante o período do estudo, a taxa de inclusão (68,6%) foi elevada (idade média 68±10 anos e 70% dos participantes do género masculino). A indicação para ICP foi uma síndome coronária aguda em 58% dos casos e 63% tinham doença multivaso (Score SYNTAX score 15,6±10,7; comprimento e diâmetro médios dos stents implantados de 26,3±14,8 e 3,0±1 mm, respetivamente). Entre os doentes sem terapêutica anti-coagulante oral concomitante, apenas 49,8% receberam indicação para inibidores potentes do recetor P2Y12 e os regimes de curta duração (<6 meses) de terapêutica anti-plaquetar dupla foram recomendados em 26,5%, sem diferenças de acordo com o risco hemorrágico avaliado pelo score PRECISE-DAPT (24,6% versus 29,7%, nos doentes de risco baixo versus elevado, respetivamente; p<0,125). Naqueles sob anti-coagulação oral, 62,6% tiveram indicação para regimes <30 dias, ou apenas terapêutica anti-coagulante (13,6%). Anti-agregação dupla prolongada (>12 meses) foi planeada em apenas 0,7% de todos os casos e em 1,2% dos doentes tratados no contexto de SCA. A complexidade da intervenção, mas não a extensão da doença coronária, associou-se a duração da terapêutica anti-plaquetar. A duração da diabetes foi reportada em >6 anos por 57% dos doentes (HbA1c 7,6±1,7%) e 12% apresentavam complicações microangiopáticas no momento da inclusão. A utilização de fármacos modificadores do prognóstico como os inibidores da SGLT2 (28%) e dos análogos da GLP-1(3%) foi relativamente baixa na admissão.
ConclusõesRegimes de dupla anti-agregação com duração entre os 6 e os 12 meses e baseados principalmente em AAS e clopidogrel foram os mais utilizados. A duração da terapêutica anti-plaquetar dupla relacionou-se essencialmente com a complexidade da intervenção e com a anti-coagulação oral. O controlo metabólico registado na inclusão foi subóptimo na população estudada, tal como a utilização de medicação anti-diabética presentemente recomenda (Clinicaltrials.gov NCT04481997).
Diabetes is a major determinant of clinical outcomes in patients with clinically overt coronary artery disease (CAD).1–4 In the setting of percutaneous coronary intervention (PCI), factors such as disease complexity and the inherently higher risk of progressive atherosclerosis and future ischemic events (including revascularization failure),5,6 should be considered when tailoring antithrombotic regimens and disease-modifying drug therapy. Concordantly, diabetes weighs in several risk scores that aim at estimating post-PCI thrombotic risk – such as the DAPT score7 – and clinicians are compelled by current recommendations to integrate these calculations (together with bleeding risk) into management decisions concerning the duration of dual antiplatelet therapy.8 Also, and importantly, in recent years, sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide-1 (GLP-1) analogues have been shown to further reduce cardiovascular morbidity and mortality in patients with diabetes and coronary disease,9–11 which was a major breakthrough in the pharmacological management of diabetes.
The uptake of current guidelines in everyday practice may however be hampered by several hurdles, such as the external validity of randomized trials that support guidelines, as well as financial and logistical constraints, especially in certain socio-economic scenarios. As a result, significant variability in treatment prescriptions may exist, which is largely unknown. Accordingly, to further address these gaps, a prospective nationwide observational study was undertaken.
ObjectivesThe study main objectives were to (1) describe the clinical characteristics of an unselected consecutive population of patients with DM undergoing PCI with stent implantation; (2) determine the anti-thrombotic regimens prescribed, regarding drugs of choice, prescribed dosage, indicated and actual duration of treatment, their main determinants and adherence, and (3) evaluate the prescription patterns of guideline-directed drug therapy for diabetes at baseline and during follow-up. Also, the incidence of major ischemic and hemorrhagic events at two years was assessed.
This paper describes the baseline inclusion data and drug prescription regimens.
MethodsStudy design and oversightThe anti-thrombotic and glucose lowering therapy in diabetic patients with coronary artery disease undergoing PCI: a prospective multicenter observational study on drug use and implications for clinical outcomes (ARTHEMIS) study, was an investigator-initiated nationwide prospective observational study, implemented at 12 medium-to-high volume PCI-capable hospitals in Portugal. A detailed description of the clinical endpoints and their definitions is provided in the Supplemental Materials.
Baseline data was collected at the time of inclusion in a dedicated e-CRF. The study was planned and reported according to the STROBE statement for reporting observational studies.12 An external contract research organization ensured the quality and reliability of the data, through regular monitoring.
Patient populationAll consecutive patients with type-2 diabetes undergoing PCI with stent implantation performed in at least one major coronary artery in the context of stable CAD or an acute coronary syndrome were screened and invited to participate. Inclusion could be considered at any time between PCI and hospital discharge. Other than balloon-only PCI, short life expectancy due to significant comorbidity (according to the opinion of the attending physician), type 1 diabetes and unwillingness to participate, no major exclusions existed. Inclusion and exclusion criteria are detailed in the online Supplemental Material.
Study procedures and definitionsFor screening and inclusion purposes, the diagnosis of type-2 diabetes was adjudicated as self-reported when already known at the time of hospital admission, and according to the 2021 criteria of the American Diabetes Association when newly diagnosed or confirmation was warranted.13 Ischemic and bleeding risks were estimated by the DAPT7 and PRECISE-DAPT Risk Scores.14
After screening, patients were treated according to local practice; PCI technique was left to the discretion of the operator, as well as subsequent medical management. The indication for PCI was reported by the local investigators and was not centrally adjudicated. As per-protocol, DAPT regimen had to be specifically indicated, including the drugs prescribed and specific planned duration. If available (according to local clinical practice in each hospital), HbA1c was registered at the index admission and at each visit.
Sample size and statistical analysisTaking the sample size of previously published studies that evaluated different strategies of DAPT duration as reference, we arbitrarily estimated that the inclusion of 1000 consecutive patients would allow for a detailed characterization of the diabetic population with CAD and the evaluation of ischemic, heart failure and hemorrhagic events throughout the two-year follow-up.
Results are reported as percentages (for dichotomous variables) and as means and standard-deviation or median and interquartile range for continuous variables, as appropriate. Normality was assessed by the Kolmogorov-Smirnoff test and by visual inspection of histograms and Q-Q plots. When necessary, comparisons were performed using the χ2 test (with Yates correction when appropriate) for categorical variables, the Student t test, the Satterthwaite test or 1-way ANOVA for continuous variables with normal distribution or the Kruskal–Wallis test for continuous variables with a non-normal distribution. When appropriate, 95% confidence intervals were calculated. Two-tailed tests of significance are reported. For all comparisons, a p-value of <0.05 was considered statistically significant. Statistical analysis was performed with IBM SPSS software version 23 (SPSS Inc, Chicago, IL).
Ethics and regulationThe study was undertaken according to good clinical practices and all patients provided written informed consent. The protocol complies with the Declaration of Helsinki and was approved by institutional review boards at each participating site. The study was registered with the Portuguese National Data Protection Committee and on ClinicalTrials.gov (identifier: NCT04481997).
Funding and sponsorshipThe study was sponsored by the Association for Research and Development of the Faculty of Medicine (AIDFM) – Cardiovascular Center of the University of Lisboa (CCUL) and had the scientific sponsorship of the Portuguese Association of Cardiovascular Intervention (APIC). It was funded as an investigator-initiated study, by an unrestricted grant from AstraZeneca (ESR-19-20188). Study coordinators and local investigation teams were fully responsible for protocol preparation, patient selection, study compliance, and data entry and analysis.
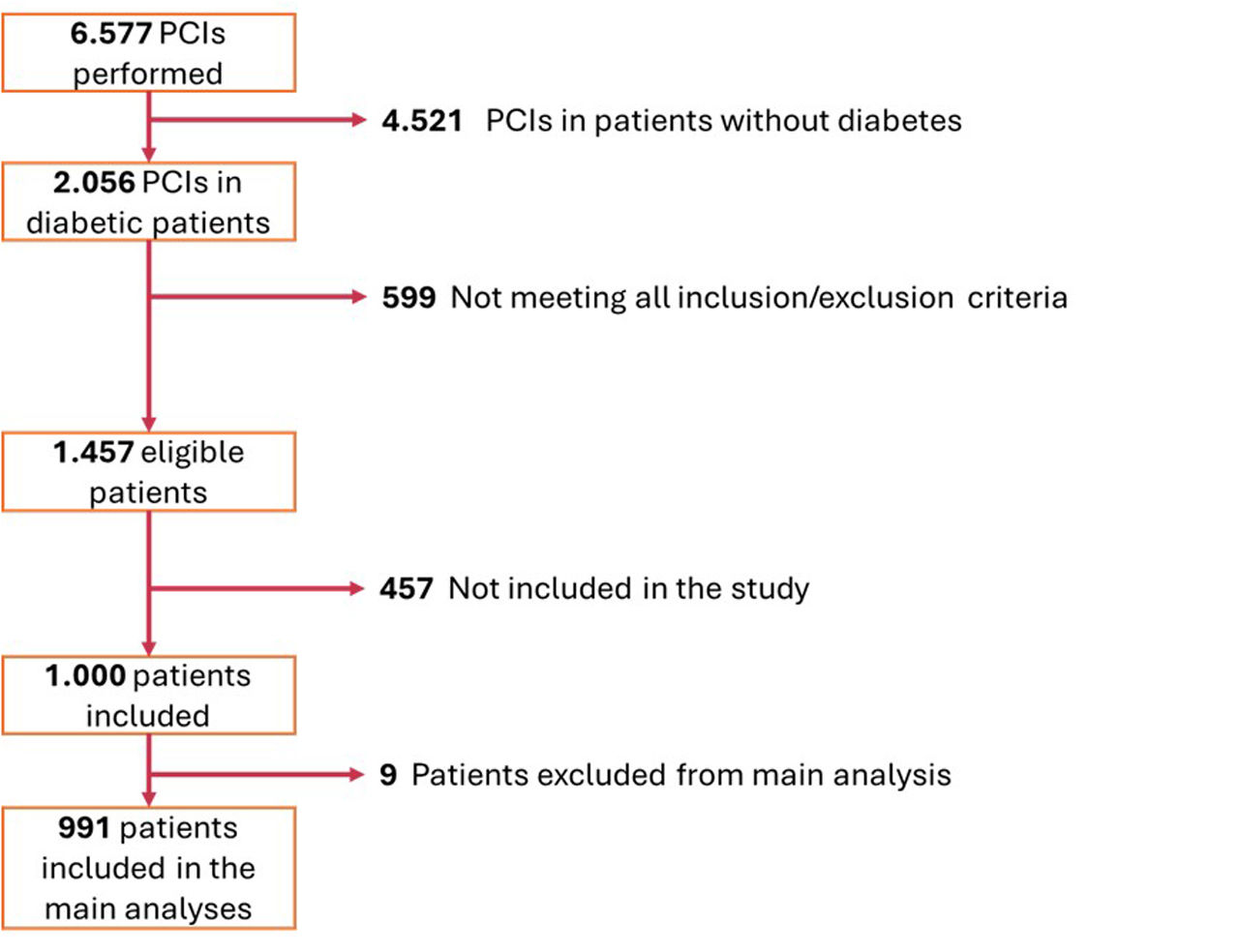
ResultsPatient inclusionBetween January and November 2022, 6557 PCI procedures were performed in the 12 participating centers, of which 2056 (31.4%) were reportedly in patients with diabetes. From the 1457 who were eligible (meeting all inclusion/exclusion criteria), 1000 were included in the study at the time of the procedure (68.6%). Of these, four were subsequently excluded because a diagnosis of diabetes was not confirmed. Of the remaining 996 patients, five died during the index hospital admission and were excluded from the analysis (Figure 1). Overall average inclusion rate was 111 patients/month. A description of inclusion rates per each participating hospital is detailed in Supplemental Material (Supplemental Figure 2 and Supplemental Table 1).
Flowchart of patient inclusion (for details, see Supplemental Figure 1).
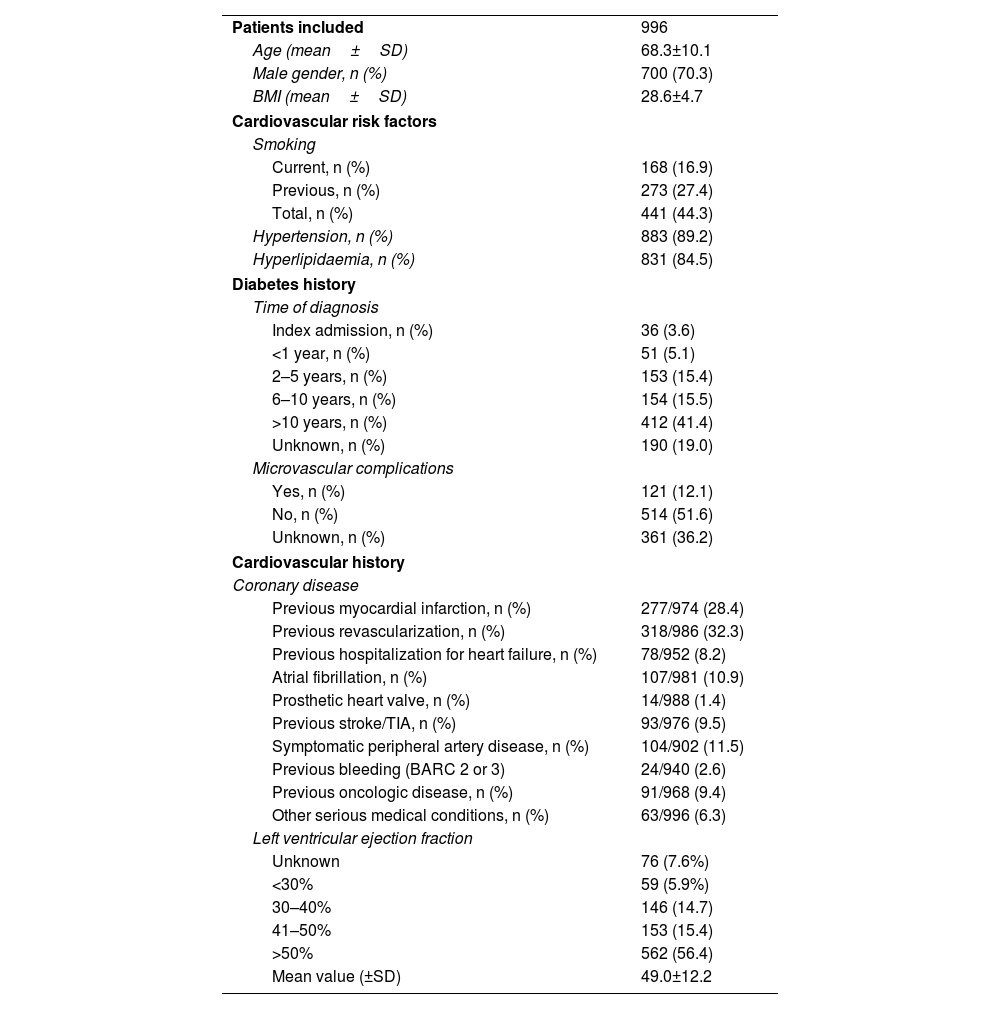
The mean age was 68±10 years-old and 70.3% of participants were male. The prevalence of classical risk factors was very high, with more than 4/5 of all patients reporting hypertension or dyslipidemia, and almost half active or past history of smoking. Also, roughly one third had clinically overt CAD (28.4% previous acute myocardial infarction and 32.3% a prior revascularization event), while mean LVEF was 49±12%, and 8.2% reported at least one prior admission due to heart failure. Baseline characteristics are detailed in Table 1.
Baseline characteristics – epidemiological data and previous history.
| Patients included | 996 |
| Age (mean±SD) | 68.3±10.1 |
| Male gender, n (%) | 700 (70.3) |
| BMI (mean±SD) | 28.6±4.7 |
| Cardiovascular risk factors | |
| Smoking | |
| Current, n (%) | 168 (16.9) |
| Previous, n (%) | 273 (27.4) |
| Total, n (%) | 441 (44.3) |
| Hypertension, n (%) | 883 (89.2) |
| Hyperlipidaemia, n (%) | 831 (84.5) |
| Diabetes history | |
| Time of diagnosis | |
| Index admission, n (%) | 36 (3.6) |
| <1 year, n (%) | 51 (5.1) |
| 2–5 years, n (%) | 153 (15.4) |
| 6–10 years, n (%) | 154 (15.5) |
| >10 years, n (%) | 412 (41.4) |
| Unknown, n (%) | 190 (19.0) |
| Microvascular complications | |
| Yes, n (%) | 121 (12.1) |
| No, n (%) | 514 (51.6) |
| Unknown, n (%) | 361 (36.2) |
| Cardiovascular history | |
| Coronary disease | |
| Previous myocardial infarction, n (%) | 277/974 (28.4) |
| Previous revascularization, n (%) | 318/986 (32.3) |
| Previous hospitalization for heart failure, n (%) | 78/952 (8.2) |
| Atrial fibrillation, n (%) | 107/981 (10.9) |
| Prosthetic heart valve, n (%) | 14/988 (1.4) |
| Previous stroke/TIA, n (%) | 93/976 (9.5) |
| Symptomatic peripheral artery disease, n (%) | 104/902 (11.5) |
| Previous bleeding (BARC 2 or 3) | 24/940 (2.6) |
| Previous oncologic disease, n (%) | 91/968 (9.4) |
| Other serious medical conditions, n (%) | 63/996 (6.3) |
| Left ventricular ejection fraction | |
| Unknown | 76 (7.6%) |
| <30% | 59 (5.9%) |
| 30–40% | 146 (14.7) |
| 41–50% | 153 (15.4) |
| >50% | 562 (56.4) |
| Mean value (±SD) | 49.0±12.2 |
Most patients underwent PCI in the setting of an ACS (20.3% ST elevation myocardial infarction and 37.6% NSTE-ACS) and 63.1% had multivessel CAD, with a mean SYNTAX score of 15.6±10.7. Despite disease extent, most patients (71%) had only one lesion treated with stent implantation. The mean number of stents per-lesion and per-patient was 1.14 and 1.57, respectively; 45% of implanted stents had a diameter <3.0 mm (mean 3.0±1.2 mm) and mean stent length was 26.3±14.8 mm (Table 2).
Baseline characteristics – coronary disease and coronary intervention.
| Indication for angiography (n=996) | |
| Chronic coronary syndrome, n (%) | 420 (42.1) |
| nST acute coronary syndrome, n (%) | 374 (37.6) |
| ST elevation acute myocardial infarction, n (%) | 202 (20.3) |
| Coronary artery disease | |
| Number of vessels with disease (n=996) | |
| 1 vessel, n (%) | 368 (36.9) |
| 2 vessels, n (%) | 334 (33.6) |
| 3 vessels, n (%) | 294 (29.5) |
| Multivessel disease, n (%) | 628 (63.1) |
| Syntax score (n=919) (mean±SD) | 15.6±10.7 |
| Percutaneous coronary intervention | |
| Number of treated lesions per patient (n=993) | |
| 1 lesion, n (%) | 705 (71.0) |
| 2 lesions, n (%) | 215 (21.7) |
| ≥3 lesions, n (%) | 73 (7.3) |
| Number of stents per patient (n=993) | |
| 1 stent (%) | 614 (61.8) |
| 2 stents, n (%) | 250 (25.2) |
| ≥3 stents, n (%) | 129 (13.0) |
| Mean number of stents per-patient | 1.57 |
| Mean number of stents per-lesion | 1.14 |
| Total stent length ([mm]; mean±SD) | 26.3±14.8 |
| Stent diameter | |
| <3.0 mm, n (%) | 705 (45.0) |
| 3.0 mm, n (%) | 423 (27.0) |
| >3.0 mm, n (%) | 431 (28.0) |
| Stent diameter ([mm]; mean±SD) | 3.0±1.2 |
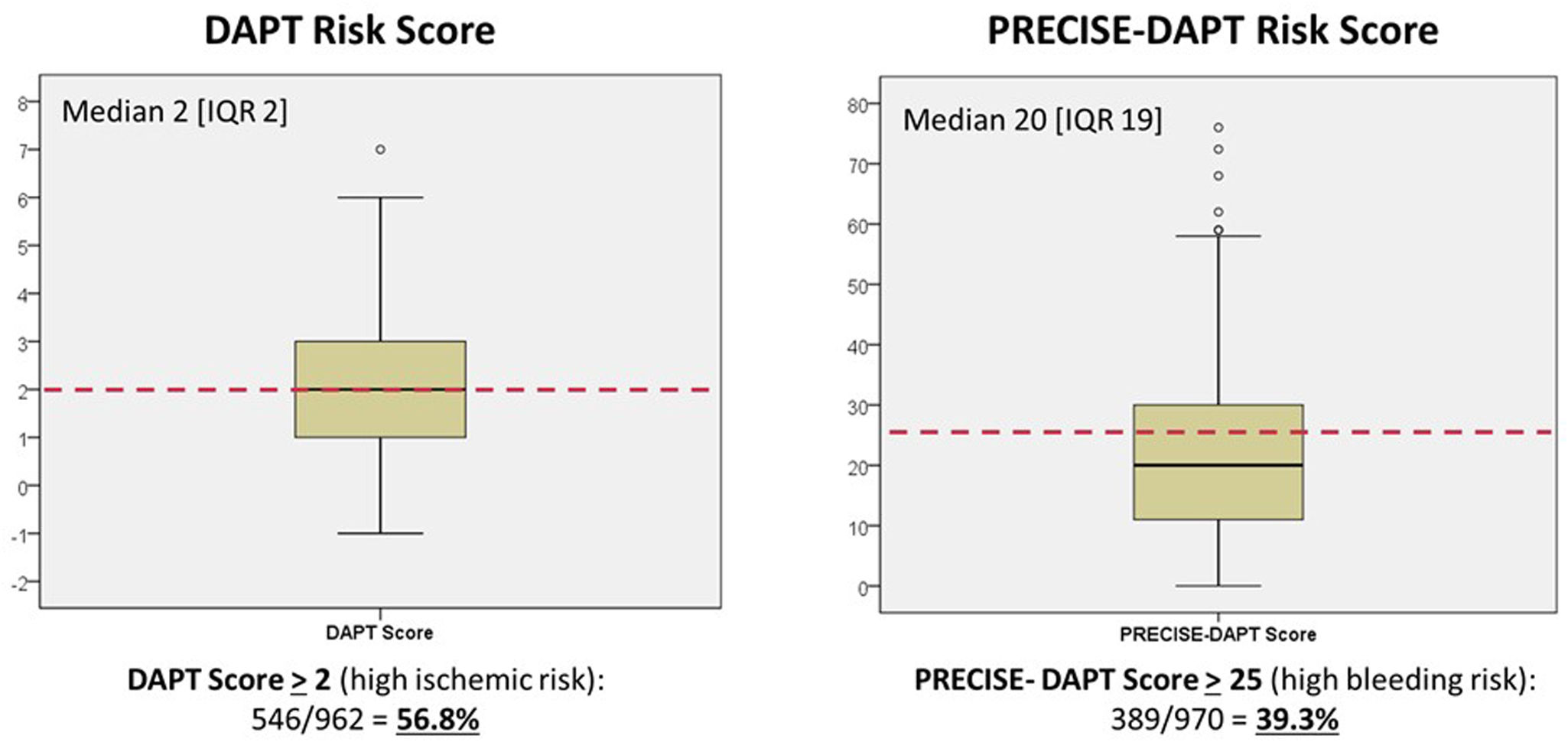
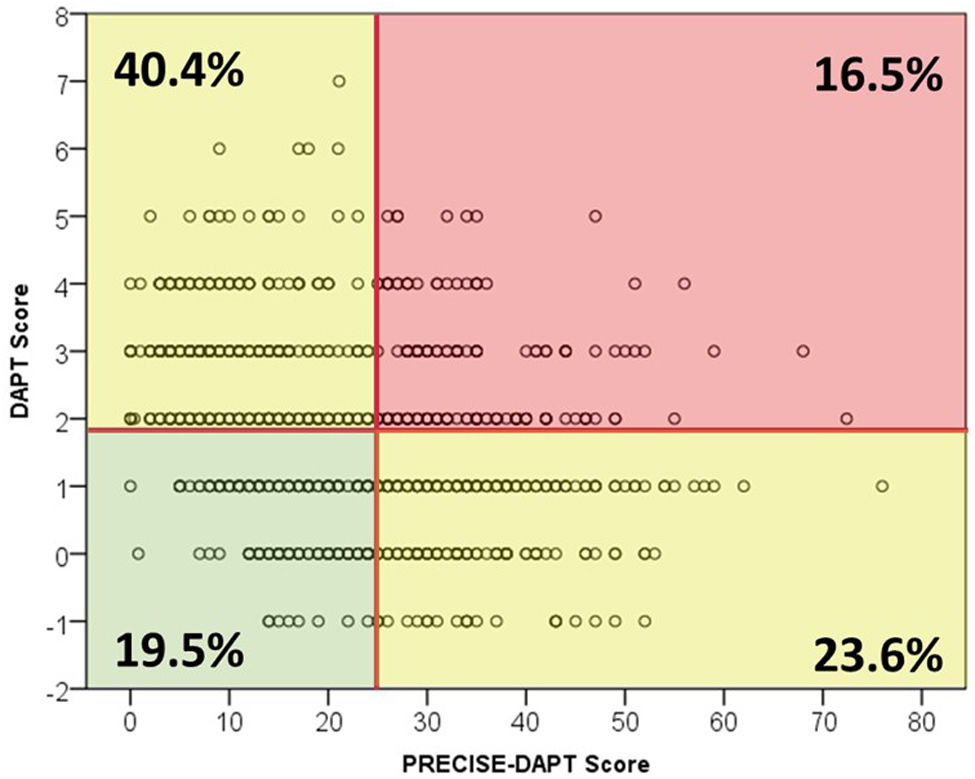
A DAPT score of 2 or greater – indicating a high ischemic risk – was present in 546 (56.9%) of the 962 patients for whom all variables were available for calculation. Likewise, the PRECISE-DAPT score was >25 (indicating a high bleeding risk) in 389 out of 970 (39.3%) (Figure 2). The distribution and relationship between ischemic and bleeding scores are shown in Figure 3 and Supplemental Figure 3. Notably, although high ischemic risk was significantly more frequent in ACS vs. stable patients (67.9% vs. 40.8%, p<0.001), the proportion of patients with high bleeding risk was roughly similar in either subgroup (38.7% vs. 41.1%, p=0.29).
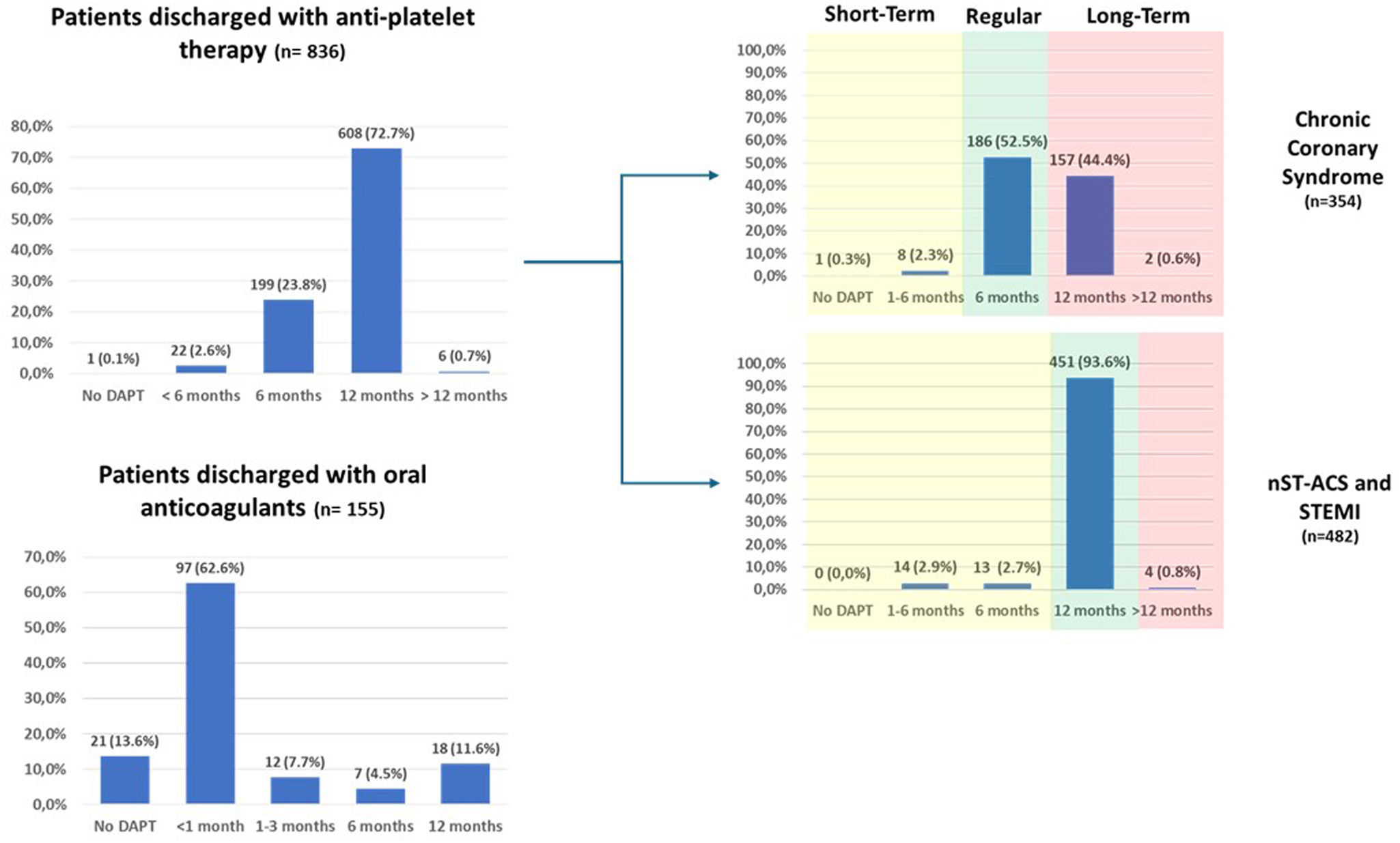
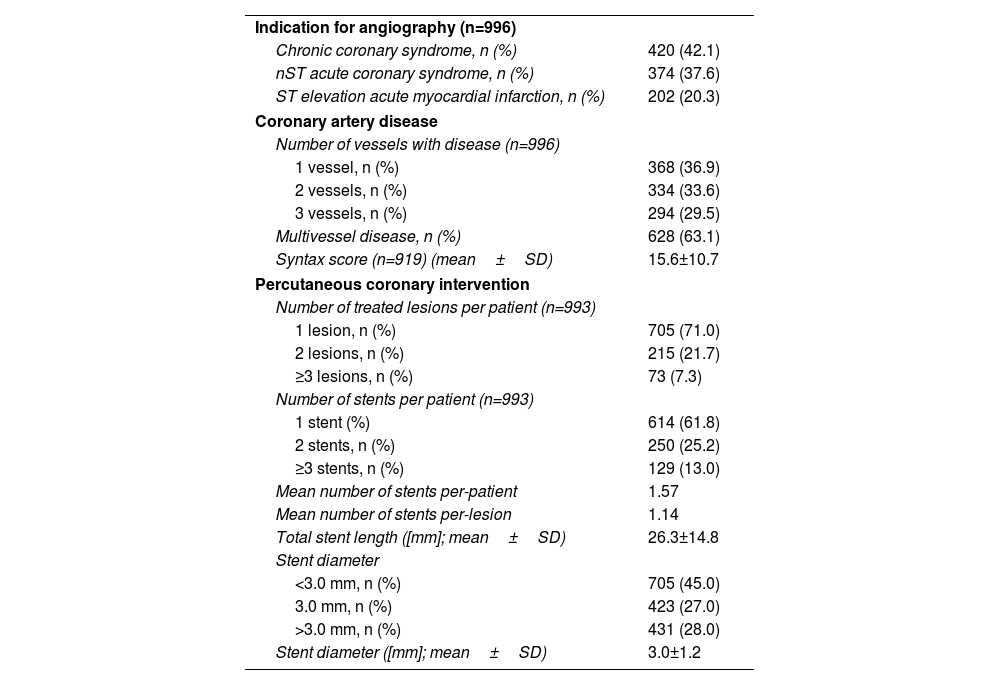
Antithrombotic therapy at admission and hospital dischargeRemarkably, 33.8% of patients were not on any anti-thrombotic treatment at the time of hospital admission – the majority of whom were ACS patients (83.1%) – while 15.9% were already on dual antiplatelet therapy (DAPT) (Supplemental Figure 4). At hospital discharge, DAPT was prescribed to 99.9% of patients without concomitant anticoagulation and to 84.5% of patients also treated with anticoagulants (Figure 4 and Supplemental Table 2). Among the former group potent P2Y12 inhibitors (ticagrelor and prasugrel) were used in only 417/836 (49.8%) (Supplemental Table 2), although, as expected, the proportion was still much higher in patients with ACS as compared to chronic coronary syndromes (CCS) (74.7% vs. 16.1%; p<0.001).
The overall proportion of patients with ACS not receiving new-generation P2Y12 antagonists was 36.3%. These patients were older (71±10 vs. 65±10 years old; p<0.001), had lower body mass index (28.2±4.3 vs. 29.2±5.0 kg/m2; p=0.007), lower creatinine clearance (68.9±31.5 ml/m2 vs 93.4±41.8 ml/min; p<0.001), were more likely to have atrial fibrillation (25% vs. 1%; p<0.001) or any indication for anticoagulation (41.3% vs. 1.4%; p<0.001), all translating into an higher bleeding risk as assessed by the PRECISE-DAPT score (26.7±13.5 vs. 17.9±12.0; p<0.001). Prior history of BARC 2 or 3 bleeding, and gender were not associated with the likelihood of potent P2Y12 inhibitor prescription. Conversely, 13.6% of patients treated in the setting of chronic coronary syndrome did receive DAPT with ticagrelor or prasugrel.
Planned duration of antithrombotic therapyIn the cohort of patients without concomitant anticoagulation, recommendation for shorter DAPT regimens (≤6 months) or no DAPT was 26.5% and did not differ significantly according to bleeding risk (24.6% vs. 29.7% in low vs. high-bleeding risk as defined by a PRECISE-DAPT score >25; p=0.125). In patients with chronic coronary syndromes, recommended DAPT durations of 6 and 12 months were 55.2% and 44.4%, respectively, while in those treated in the setting of an ACS, 12 months were the standard indication (93.6%). Prolonged DAPT (≥12 months, irrespectively of the initial indication for the PCI) was planned in only 0.7% of patients (Figure 4 and Supplemental Table 3).
When the duration of DAPT was evaluated according to clinical setting, and considering “regular” a DAPT duration of 6 or 12 months; “short-term” a DAPT duration of <6 or <12 months; and “long-term” a DAPT duration of >6 or >12 months, respectively for CCS and ACS patients, shorter durations were infrequently planned but still more likely to be prescribed in high bleeding-risk patients (6.5% vs. 2.5%, p=0.038), and longer durations were more prevalent in high ischemic risk patients (24.0% vs. 15.9%, p=0.015) (Supplemental Table 4). Still, prolonged DAPT was initially planned at discharge in only 1.2% of ACS patients with a potential indication (Supplemental Table 5).
Furthermore, age and gender did not influence planned DAPT duration, nor did the complexity of coronary disease, as assessed by the Syntax score, the presence of multivessel disease or the number of lesions (Supplemental Table 4). On the other hand, longer DAPT duration was planned more often in patients with previous myocardial infarction (29.8% vs. 15.1%, p<0.001), prior revascularization (29.9% vs. 14.3%, p<0.001) and also in cases with more complex procedures (defined by higher number of implanted stents and longer total stent length) (Supplemental Table 4). This observation was consistent for both chronic disease and ACS (Supplemental Tables 5 and 6).
Of note, in all participants also on oral anticoagulants, at least one antiplatelet agent was added, and triple therapy (DAPT+anticoagulant) was prescribed in 134/155 (86.5%) of these patients (supplemental Table 2). In almost all cases (128/134, 95.5%), the preferred DAPT regimen was aspirin and clopidogrel.
Diabetes staging and characterizationSelf-reported duration of DM was longer than five years in 57% of participants, 12% had known microangiopathic involvement at inclusion, and 3.6% were newly diagnosed during the index admission (Table 1). Mean hemoglobin A1c and fasting glucose levels were 7.6±1.7% and 164±66 mg/dl, respectively. Microangiopathic involvement at inclusion was known in 12% of patients.
Notably, only 28% and 3% of patients were taking SGLT2 inhibitors and GLP-1 analogues on admission, respectively, a proportion that increased significantly for the former (51%), but not for the latter (4%) at discharge (Supplemental Figure 5).
DiscussionTo the best of our knowledge, this is the first study to specifically characterize prospectively a contemporary population of patients with type-2 diabetes and concomitant obstructive CAD undergoing stent implantation, in terms of clinical and cardiovascular risk profiles, as well as the most common approaches to pharmacological management. Our main findings can be summarized as follows: (1) both ischemic and bleeding risks were relatively high, as assessed by the DAPT and PRECISE-DAPT risk scores; (2) PCI complexity, but not CAD extent, was associated with DAPT duration; (3) the adherence to single antiplatelet regimens from inception in high bleeding risk patients was relatively low; and (4) metabolic control was off-target and guideline-directed treatment for diabetes was underused at hospital admission, although it improved at discharge.
Representative real-world dataRegistry data provides an opportunistic snapshot on how results of randomized trials are translated to everyday practice through guideline uptake. Our registry has the virtue of including centers across the entire country, encompassing a wide range of socio-economic and logistic scenarios thus enabling a solid representation of the real world. Importantly, the information concerning almost 70% of all eligible patients has been captured, although the main reason for non-inclusion was unclear (operator decision or not reported). Interestingly though, baseline characteristics of our patients are remarkably similar to those of patients with diabetes included in the THEMIS (ticagrelor in patients with stable coronary disease and diabetes) randomized trial, except for a lower incidence of prior revascularization and lower ejection fraction.15
Antithrombotic and antiplatelet regimensIt is noteworthy that despite the high-risk profile of our population, over 1/3 of patients were not on any antithrombotic treatment by the time of hospital admission. These patients were less likely to have classical risk factors (such as hypertension and high cholesterol, but not smoking history), clinically overt vascular disease, prior admissions due to heart failure and atrial fibrillation, but there were no differences concerning gender and age.
At hospital discharge, the overall adherence to current recommendations in both chronic coronary syndrome and ACS patients seemed to be quite straightforward, although a significant proportion of patients with ACS were not discharged on a new P2Y12 inhibitor. These patients were older and had a higher bleeding risk, although recent guidelines do not in fact encourage this approach.16 On the other hand, PCI complexity was the main modulator of total prescribed DAPT duration. Trials assessing prolonged DAPT regimens have not been stratified by CAD complexity at baseline, just after PCI, and the DAPT score was developed in patients that have completed a standard duration regimen without events.7 Still, variables related to procedural risk and complexity have some weight in the score and a post-hoc patient-level metanalysis of randomized trials showed a significant interaction between the benefits of prolonged DAPT duration and PCI complexity.17
DAPT regimens and bleeding riskTrials performed in patients at high risk of bleeding undergoing PCI have suggested that short DAPT regimens are safe, whether or not on a background of direct oral anticoagulants. Despite individual trials have been formally underpowered to specifically assess between-group differences regarding thrombotic events (such as stent thrombosis), safety has been confirmed in large series and metanalysis.18,19 Operators have seemingly welcomed these findings as demonstrated by the high adoption of short protocols (<6 months) particularly in patients with a concomitant indication for anticoagulation, but also – although to a lesser extent – in those with high PRECISE-DAPT score and no-anticoagulation. Ongoing follow-up will further inform on clinical outcomes of these strategies on a real word setting.
Glucose-lowering therapyGuidelines recommend a HbA1c<7% as a metabolic control target in patients with diabetes and CAD.20 Although glycemic levels are associated with cardiovascular outcomes, the most widely used drugs for diabetes for decades – namely metformin, sulfonylureas and dipeptidyl-peptidase-4 inhibitors – have either not been specifically tested or have failed to consistently reduce ischemic events and mortality, despite improving glucose levels.11,21 Recently, SGLT2 inhibitors and GLP-1 analogues have gained preponderance as guideline-recommended first line therapy, driven by significantly improvements in cardiovascular morbidity and mortality, even in patients without overt diabetes.20,22 Although in our cohort, mean HbA1c was within range of prior randomized trials performed in patients with DM and known CAD,15,23 optimal metabolic control rates (Hb A1c<7%) were worse than those reported both in the first diabetes prevalence study in Portugal (PREVADIAB study)24 and in the First National Health Examination Survey – INSEF 201525 (41% vs. 69% and 63%, respectively). This discrepancy most likely stems from differences in the targeted patients, given that the aforementioned surveys were aimed at studying broader populations with known and unknow diabetes, rather than selected individuals with clinically overt atherosclerotic disease, inherently more likely to have advanced diabetes and poorer metabolic control. Still, less than one third of patients were taking SGLT2 inhibitors by the time of hospital admission, and the proportion of those medicated with GLP-1 analogues was marginal. Despite the improvement at hospital discharge, prescription rates, especially for SGLT2 inhibitors, were still suboptimal. Altogether, this may be a reflection of some unwarranted perception of low tolerability to the drug in the real-life setting, impaired awareness of potential benefits by clinicians, worries about a low absolute benefit at the individual patient level, and, not less importantly, cost constraints.
LimitationsOur work has the inherent limitations of observational studies. It is not possible to reliably measure the potential impact of the Hawthorne effect and its influence on prescription patterns. Considering that the calculation of risk scores (both hemorrhagic and ischemic) may not always be routine practice, it is possible that their knowledge by operators and/or attending physicians may have induced some behavioral change, by increasing the chance of changing standard DAPT duration (as compared to empirical clinical judgment alone).
Despite recommendations being made to include all consecutive cases meeting study criteria, over one third of eligible patients were not enrolled, mostly for unknown reasons. As data on this group was not systematically collected, it is not possible to firmly assess any potential biases resulting from inadvertent selection. Still, as previously mentioned, the baseline characteristics of our population are similar to other studies focusing on the same patient subset, which is reassuring of the external validity of our study.
Finally, for most variables including the diagnosis and duration of diabetes, investigators relied on self-reported data. Nevertheless, although confirmation of diabetes by laboratory testing was not protocol-mandated, only a minority of patients were excluded because of unconfirmed diabetes.
ConclusionsIn this real-world population of patients with type-2 diabetes and obstructive CAD undergoing PCI with stent implantation, both ischemic and bleeding risks were relatively high, as assessed by the DAPT and PRECISE-DAPT scores. Recommendations for DAPT duration were mostly in line with current guidelines. Clinical setting (ACS vs. CCS), the need for concomitant oral anticoagulation and PCI complexity (but not CAD extent) were the main correlates of DAPT duration. Average metabolic control was off-target and guideline-directed treatment for diabetes was underused at baseline visit, although it improved by the time of hospital discharge.
Ethical approvalThe protocol complies with the Declaration of Helsinki and was approved by institutional review boards at each participating site.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to acknowledge the CRO CETERA team (Inês Zimbarra Cabrita, Susana Silva and Carolina Rodrigues) for the implementation and management of the study in Portugal.