Heart failure (HF) is associated with significant cardiovascular morbidity and mortality across all categories (with preserved, mid-range or reduced left ventricular ejection fraction). Recently introduced drug classes, especially sodium-glucose co-transporter 2 (SGLT2) inhibitors and angiotensin receptor-neprilysin inhibitors (sacubitril/valsartan), have provided significant cardiovascular benefits for subjects with HF, as shown in recent hallmark trials, and represent a step forward in the therapeutic management of these patients.1,2 These drug classes have accordingly been prioritized in the treatment algorithm of patients with HF, especially for those with reduced ejection fraction, according to the latest HF guidelines published by the European Society of Cardiology.3 At the same time, mineralocorticoid receptor agonists (MRAs) constitute a drug class with established cardioprotective action in patients with HF. We therefore sought to determine whether combining SGLT2 inhibitors or sacubitril/valsartan and MRAs leads to a further decrease in the risk for surrogate cardiovascular endpoints among patients with HF.
We searched PubMed for randomized controlled trials (RCTs) assessing the effect on surrogate cardiovascular endpoints of SGLT2 inhibitors and sacubitril/valsartan versus placebo or active comparator among subjects with HF. We extracted data corresponding to subgroup analyses according to prior treatment with MRAs. We set as the primary endpoint the composite of hospitalization for HF or cardiovascular death.
Two reviewers (D.P. and C.P.) independently extracted data of interest from the eligible studies. As we assessed only a dichotomous variable, differences were calculated with the use of risk ratios (RR) with 95% confidence interval (CI), after implementation of the Mantel-Haenszel random effects model. Statistical heterogeneity among studies was assessed by means of I2 statistics. All analyses were performed at the 0.05 significance level.
We finally included five RCTs in our analysis, of which three assessed the cardiovascular efficacy and safety of SGLT2 inhibitors compared to placebo and two addressed the cardiovascular efficacy and safety of sacubitril/valsartan versus enalapril or valsartan.4–8
We pooled data on a total of 14 462 subjects with HF assigned to either an SGLT2 inhibitor or placebo and 13 195 subjects with HF randomized to either sacubitril/valsartan or a renin-angiotensin system (RAS) blocker.
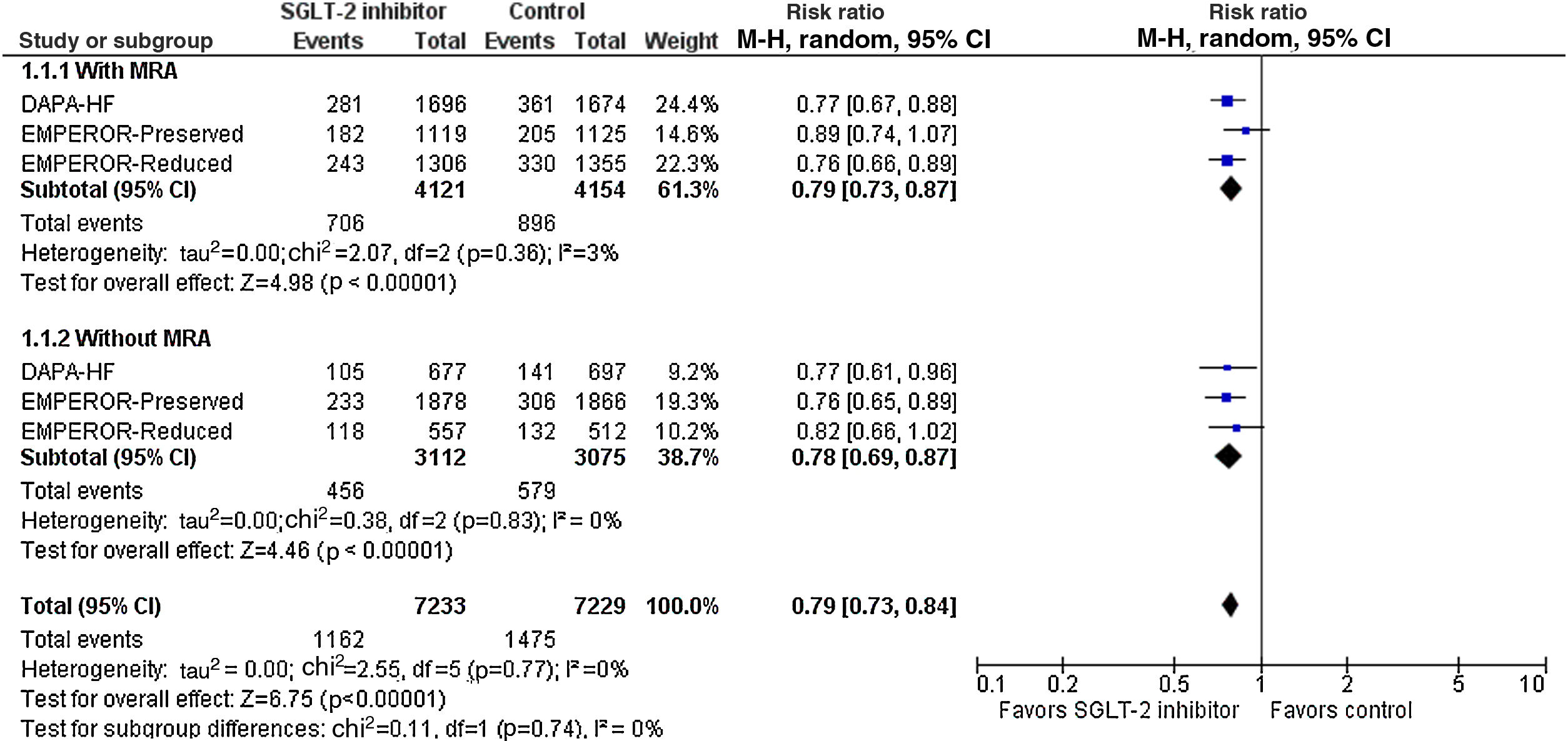
As shown in Figure 1, prior treatment with an MRA did not affect the cardiovascular efficacy of SGLT2 inhibitors compared to placebo: patients receiving a combination of an SGLT2 inhibitor and an MRA experienced a 21% decrease in the risk for the primary composite endpoint (RR=0.79, 95% CI: 0.73-0.87, I2=0%), while patients without prior use of MRAs assigned to an SGLT2 inhibitor experienced a 22% decrease in the risk for the same endpoint (RR=0.78, 95% CI: 0.69-0.87, I2=0%). No difference between subgroups was detected (p=0.74).
Effect of sodium-glucose co-transporter 2 inhibitors compared to placebo on the risk for the primary composite endpoint according to prior use of mineralocorticoid receptor agonists. CI: confidence interval; LCZ696: sacubitril/valsartan; M-H: Mantel-Haenszel; MRA: mineralocorticoid receptor agonist.
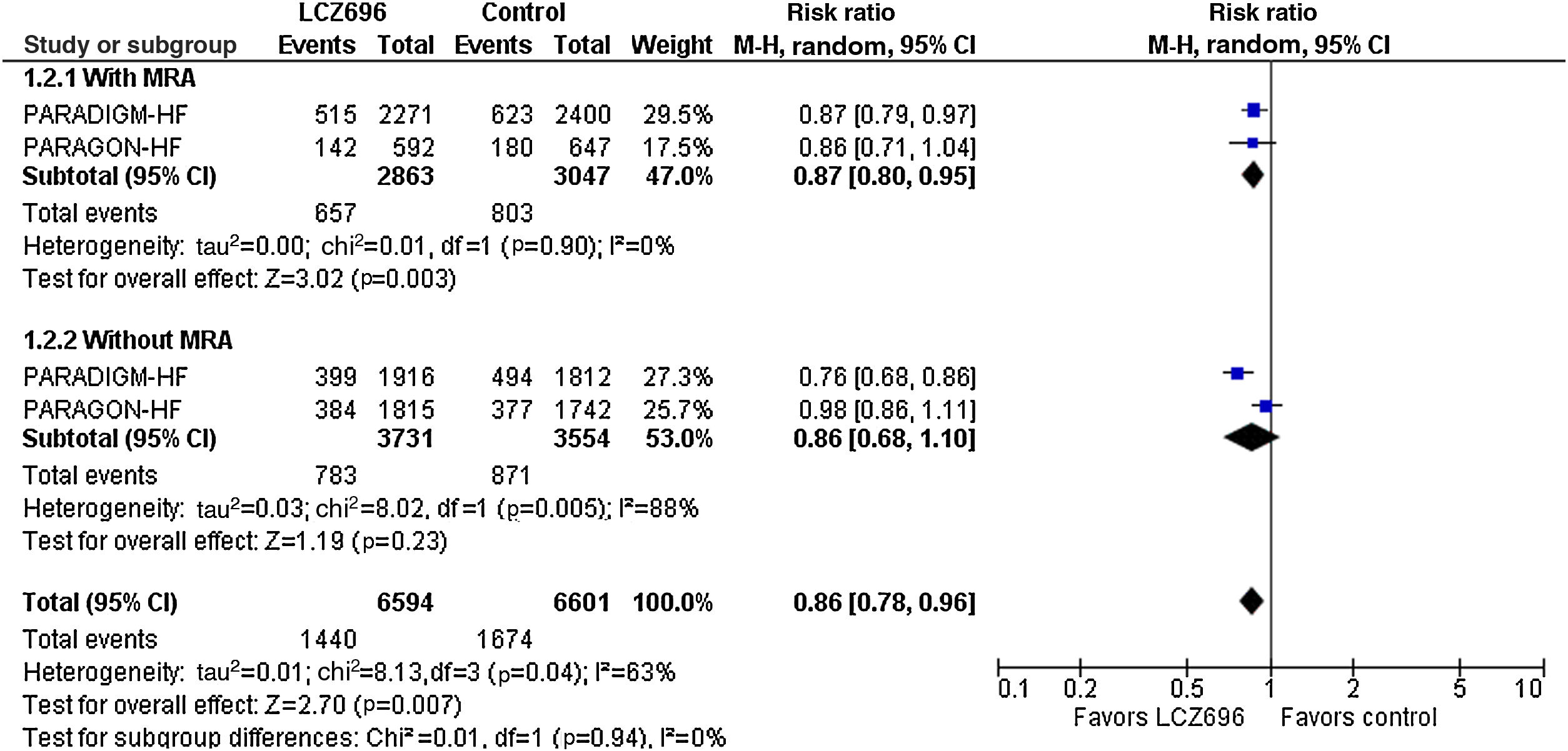
When we assessed the cardiovascular efficacy of sacubitril/valsartan versus RAS blockers (Figure 2), we found that the combination of sacubitril/valsartan and an MRA compared to the combination of a RAS blocker and an MRA led to a significant 13% decrease in the risk for the primary composite endpoint (RR=0.87, 95% CI: 0.80-0.95, I2=0%). However, among patients without prior use of MRAs, sacubitril/valsartan was not superior to RAS blockers in decreasing the risk for cardiovascular death or hospitalization for heart failure (RR=0.86, 95% CI: 0.68-1.10, I2=88%). Once again, no subgroup difference was detected (p=0.94).
Effect of sacubitril/valsartan compared to active comparator on the risk for the primary composite endpoint according to prior use of mineralocorticoid receptor agonists. CI: confidence interval; M-H: Mantel-Haenszel; MRA: mineralocorticoid receptor agonist; SGLT2: sodium-glucose co-transporter 2.
Overall, although reasonable, a treatment combination of SGLT2 inhibitors or sacubitril/valsartan with MRAs does not provide additional cardiovascular benefits for patients with HF with reduced or preserved ejection fraction. Potentially synergistic effects of these drug classes are yet to be confirmed.
Conflicts of interestThe authors have no conflicts of interest to declare.