Left ventricular reverse remodeling (LVRR) is strongly related to the long-term prognosis of patients undergoing cardiac resynchronization therapy (CRT). The aim of this study was to assess the long-term clinical outcome of patients without LVRR at six months after CRT implantation and to determine the prognostic impact of clinical response in this population.
MethodsWe analyzed 178 consecutive patients who underwent successful CRT device implantation (age 64±11 years; 69% male; 89% in New York Heart Association [NYHA] functional class III; 35% with ischemic cardiomyopathy). Clinical status and echocardiographic parameters were determined before and six months after CRT implantation. We identified those without criteria for LVRR (≥10% increase in left ventricular ejection fraction with ≥15% reduction in left ventricular end-systolic diameter compared to baseline). Clinical responders were defined by a sustained improvement of at least one NYHA functional class.
ResultsAt six-month assessment after CRT, 109 (61%) patients showed LVRR. During a mean follow-up of 56±21 months, 47 (26%) patients died, with higher mortality in the group without LVRR (36% vs. 20%, p=0.023). Clinical response was greater in patients with LVRR (88% vs. 55%, p<0.001). In patients without LVRR, clinical response to CRT was the strongest independent predictor of survival (hazard ratio: 0.120; 95% confidence interval: 0.039-0.366; p<0.001).
ConclusionAlthough patients without LVRR six months after CRT implantation had a worse prognosis, with higher all-cause mortality, clinical response can be an independent predictor of survival in this population.
A remodelagem reversa do ventrículo esquerdo (RRVE) tem sido fortemente relacionada com o prognóstico em longo prazo de doentes submetidos à terapia de ressincronização cardíaca (TRC). O objetivo deste estudo foi avaliar o desfecho clínico a longo prazo de doentes sem RRVE aos seis meses após a implantação de TRC e definir o impacto prognóstico da resposta clínica nessa população.
MétodosForam analisados 178 doentes submetidos à implantação de TRC (64±11 anos, 69% do sexo masculino, 89% da classe funcional III da New York Heart Association (NYHA), 35% com cardiomiopatia isquémica). O estadio clínico e a avaliação ecocardiográfica foram feitos antes e após seis meses de TRC. Foram identificados aqueles que não tinham critérios de RRVE (aumento ≥ 10% na fração de ejeção com uma redução de ≥ 15% na dimensão sistólica do ventrículo esquerdo). Os respondedores clínicos foram definidos por uma melhoria sustentada de pelo menos uma classe funcional NYHA.
ResultadosAos seis meses de avaliação após TRC, 109 (61%) doentes apresentaram RRVE. Durante um seguimento médio de 56±21 meses, 47 (26%) doentes morreram, com maior mortalidade no grupo sem RRVE (36% versus 20%, p=0,023). A resposta clínica foi maior no grupo de doentes com RRVE (88% versus 55%, p<0,001). Em doentes sem RRVE, a resposta clínica à TRC foi o maior preditor independente de sobrevida (hazard ratio: 0,120; IC95%: 0,039-0,366; p<0,001).
ConclusãoEmbora doentes sem RRVE seis meses após a implantação da TRC apresentem um pior prognóstico com maior taxa de mortalidade por todas as causas, a resposta clínica pode ser um preditor independente de sobrevida nessa população.
atrioventricular
body mass index
cardiac resynchronization therapy
heart failure
left bundle branch block
left ventricular end-diastolic diameter
left ventricular ejection fraction
left ventricular end-systolic volume
left ventricular reverse remodeling
New York Heart Association
interventricular
Cardiac resynchronization therapy (CRT) is recommended by current guidelines for symptomatic heart failure (HF) with left ventricular ejection fraction (LVEF) ≤35% and prolonged QRS interval.1,2 CRT is effective in improving HF symptoms, exercise capacity, quality of life and cardiac function, as well as reducing HF hospitalizations and death.3–8 Trials have assessed the efficacy of CRT by means of improvement in clinical status and/or reduction in left ventricular end-systolic volume (LVESV) at mid-term follow-up.9–12 Clinical and echocardiographic responses to CRT may not coincide, but left ventricular reverse remodeling (LVRR) is considered a powerful indicator of clinical outcomes.13–15 Up to 40% of patients will not experience significant reduction in left ventricular chamber size and are defined as CRT non-responders.16 Although this population has a poor prognosis, little is known about the factors that influence their outcomes. The aim of this study was to assess the long-term clinical outcome of patients without LVRR at six months after CRT implantation and to determine the prognostic impact of clinical response in this population.
MethodsThis was a single-center study of patients who underwent successful CRT defibrillator device implantation between 2004 and 2012, a total of 178 consecutive CRT recipients. Patient data were prospectively collected in our cardiology department's information system and analyzed retrospectively. Patients were selected for CRT if they met currently recommended criteria: (1) LVEF ≤35%; (2) symptoms of HF, defined as New York Heart Association (NYHA) class II-IV despite optimal medical therapy; and (3) QRS duration ≥120 ms. Patients were classified as ischemic in the presence of significant coronary artery disease (>50% stenosis of two or more epicardial vessels or the left main, or >50% stenosis of the proximal left anterior descending coronary artery on coronary angiography, and/or a history of previous myocardial infarction or myocardial revascularization). All other patients were classified as non-ischemic. Leads were placed transvenously, via the subclavian and cephalic route, using fluoroscopy to visualize their progression and location. The right ventricular lead was positioned in the apex or mid septum. The left ventricular lead was placed with an over-the-wire system in a posterolateral or lateral tributary vein of the coronary sinus depending on ability to cannulate the veins, pacing threshold, or diaphragmatic stimulation. The standard settings included an atrioventricular (AV) delay of 100 ms (sensed) and 130 ms (paced), with DDD or DDDR mode and standard lower (50 beats/min) and upper (120-130 beats/min) pacing rates. Extensive demographic and clinical data, including mortality, NYHA class and hospitalization for worsening HF were collected from medical records. Clinical response to CRT was defined as a sustained improvement of ≥1 NYHA functional class at six-month follow-up. Transthoracic two-dimensional echocardiographic information was assessed at baseline and six months after CRT device implantation. LVRR was defined as an increase of ≥10% in LVEF over baseline combined with ≥15% reduction in left ventricular end-systolic diameter (LVESD). Cardiac structure and function were assessed using a commercially available ultrasound system (Vivid-7 and Vivid-E9; GE Vingmed Ultrasound, Horten, Norway) equipped with a 3.5-MHz transducer. LVESD, left ventricular end-diastolic diameter (LVEDD) and LVEF were determined according to standard techniques and digitally stored for offline analysis in cine-loop format. Echocardiographic CRT optimization was performed if patients presented AV and/or interventricular (VV) dyssynchrony.17 Optimization was based on the iterative method, analyzing changes in left ventricular inflow and outflow, while AV and/or VV delays were changed in 10- or 20-ms steps.
Follow-up data were obtained by review of medical records, outpatient clinic visits, and telephone contact. Ethics committee and hospital permission were obtained from the appropriate local authorities.
Statistical analysisData are expressed as mean ± standard deviation for continuous variables and as frequencies and percentages for categorical variables. Data distribution was tested for normality using the Kolmogorov-Smirnov or Shapiro-Wilk test as appropriate. Missing patient-level covariates were assumed to be missing and no imputation was performed. Comparisons of baseline characteristics and outcomes were performed using the chi-square test or Fisher's exact test, as appropriate, for categorical variables and the Student's t test or the Mann-Whitney test for continuous variables. Cumulative event rates after CRT device implantation were calculated using the Kaplan-Meier method and the population without LVRR was classified according to clinical response. Log-rank tests for time-to-event data with respect to all-cause mortality were used for statistical comparison between the patient groups. Multivariate Cox proportional hazards models were constructed with backward selection to identify independent predictors of all-cause mortality. All significant univariate clinical and echocardiographic predictors at baseline and hospitalizations for HF during follow-up were entered in the multivariate model as covariates. All statistical tests were two-sided, and a p-value <0.05 was considered significant. SPSS version 21 software (IBM SPSS Inc., Chicago, IL) was used for computation.
ResultsBaseline patient characteristicsThe study population consisted of 178 consecutive patients who underwent successful CRT device implantation (age 64±11 years; 69% male). Baseline patient characteristics are shown in Table 1. Sixty-nine patients (39%) failed to show an increase of ≥10% in LVEF and ≥15% reduction in LVESD at six-month follow-up (non-LVRR group). The majority of the cohort (65%) had non-ischemic cardiomyopathy.
Baseline clinical and echocardiographic characteristics.
| Baseline | All patients (n=178) | LVRR (n=109) | No LVRR (n=69) | p |
|---|---|---|---|---|
| Age, years, mean ± SD | 64±11 | 64±11 | 64±10 | 0.880 |
| Male gender, n (%) | 123 (69) | 66 (61) | 57 (83) | 0.003 |
| Ischemic etiology, n (%) | 62 (35) | 28 (26) | 34 (49) | 0.002 |
| NYHA class, mean ± SD | 2.9±0.4 | 3.0±0.3 | 2.9±0.5 | 0.010 |
| NYHA class ≥III, n (%) | 165 (93) | 106 (97) | 59 (86) | 0.016 |
| Weight, kg, mean ± SD | 76±13 | 76±14 | 75±13 | 0.609 |
| BMI, kg/m2, mean ± SD | 27±4 | 28±4 | 26±4 | 0.042 |
| Obesity, n (%) | 44 (25) | 33 (30) | 11 (16) | 0.058 |
| Hypertension, n (%) | 136 (76) | 86 (79) | 50 (73) | 0.358 |
| Dyslipidemia, n (%) | 100 (56) | 64 (59) | 36 (52) | 0.433 |
| Diabetes, n (%) | 59 (33) | 36 (33) | 23 (33) | 1.000 |
| History of smoking, n (%) | 59 (33) | 26 (24) | 33 (48) | 0.004 |
| COPD, n (%) | 33 (19) | 17 (16) | 16 (23) | 0.303 |
| Previous heart surgery, n (%) | 35 (20) | 14 (13) | 21 (30) | 0.006 |
| Prosthetic valve, n (%) | 15 (8) | 8 (7) | 7 (10) | 0.584 |
| Creatinine, mg/dl, mean ± SD | 1.17±0.55 | 1.13±0.52 | 1.26±0.59 | 0.170 |
| CrCl, ml/min/1.73 m2, mean ± SD | 75±33 | 77±34 | 70±30 | 0.164 |
| CrCl <60 ml/min/1.73 m2, n (%) | 61 (34) | 29 (27) | 32 (46) | 0.017 |
| Sinus rhythm, n (%) | 108 (61) | 79 (73) | 29 (42) | <0.001 |
| LBBB, n (%) | 147 (83) | 97 (89) | 50 (72) | 0.007 |
| QRS duration, ms, mean ± SD | 167±30 | 170±29 | 162±32 | 0.104 |
| QRS duration >150 ms, n (%) | 129 (72) | 81 (74) | 47 (68) | 0.191 |
| LVEDD, mm, mean ± SD | 74±10 | 72±10 | 77±9 | 0.003 |
| LVESD, mm, mean ± SD | 56±12 | 51±12 | 63±11 | <0.001 |
| LVEF, %, mean ± SD | 25±7 | 26±7 | 24±6 | 0.037 |
| ICD, n (%) | 163 (92) | 101 (93) | 62 (90) | 0.584 |
| Clinical response, n (%) | 134 (75) | 96 (88) | 38 (55) | <0.001 |
| Follow-up, months, mean ± SD | 56±31 | 72±27 | 35±21 | <0.001 |
| All-cause mortality, n (%) | 47 (26) | 22 (20) | 25 (36) | 0.023 |
| Cardiac death, n (%) | 21 (12) | 5 (5) | 16 (23) | 0.001 |
| Hospitalization for HF, n (%) | 45 (25) | 12 (11) | 33 (48) | <0.001 |
| All-cause mortality and/or hospitalization for HF, n (%) | 70 (39) | 31 (28) | 39 (56) | <0.001 |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; CrCl: creatinine clearance; HF: heart failure; ICD: implantable cardioverter-defibrillator; LBBB: left bundle branch block; LVEDD: left ventricular end-systolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-diastolic diameter; LVRR: left ventricular reverse remodeling; NYHA: New York Heart Association.
Regarding differences between the groups, patients without LVRR tended to be male (83% vs. 61% p=0.003), to have ischemic cardiomyopathy (49% vs. 26%, p=0.002) and to show more severe baseline HF (NYHA ≥III 83% vs. 97%, p=0.01). Also, no LVRR after CRT was associated with smoking (47% vs. 25%, p=0.004), lower body mass index (BMI) (26 vs. 28 kg/m2, p=0.042), previous heart surgery (30% vs. 13%, p=0.006), renal dysfunction (46% vs. 27%, p=0.017), higher LVEDD (77 vs. 72 mm, p=0.003) and LVESD (63 vs. 51 mm p<0.001), and reduced LVEF (24% vs. 26%, p=0.037). On the other hand, CRT response was associated with sinus rhythm (73% vs. 42%, p<0.001) and with left bundle branch block (LBBB) (89% vs. 72%, p=0.007).
Clinical outcomes in the overall populationDuring a mean follow-up of 56±21 months, 47 (26%) patients died, 21 (45%) due to cardiac causes, 45 (25%) patients were hospitalized due to HF, and 70 (39%) patients died or were hospitalized due to HF.
Five-year overall mortality was 39.2%, two-year mortality was 12.6% and one-year mortality was 6.7%. Annual mortality was 7.8% overall. The group with no LVRR showed higher mortality (36% vs. 20%, p=0.023), with more deaths of cardiac cause (23% vs. 5%, p=0.001), and higher rates of hospitalizations due to HF (48% vs. 11%, p<0.001). Clinical response was found to be greater in the LVRR group (88% vs. 55%, p<0.001).
Patients without left ventricular reverse remodelingThere were thirty-eight patients (55%) with an improvement of ≥1 NYHA functional class (clinical response to CRT) among the 69 patients without LVRR. Mean NYHA class improved from 2.86±0.46 at implantation to 2.19±0.71 at six-month follow-up (p<0.001, 95% confidence interval [CI], 0.50-0.83). No difference was observed between ischemic and nonischemic cardiomyopathy (p=0.81). Comparison of clinical and echocardiographic data between the two groups (clinical responders vs. non-responders among patients without LVRR) at six-month follow-up are shown in Table 2. There were no differences between the groups in age, gender, baseline NYHA class, BMI, history of diabetes or smoking, atrial fibrillation, renal dysfunction, previous heart surgery, prevalence of LBBB, QRS duration, left ventricular dimensions or systolic function, baseline mitral regurgitation or echocardiographic CRT optimization during follow-up. Twenty-five (36%) patients died, with higher mortality in the non-responders (58% vs. 18%, p=0.001). Thirty-three patients (48%) were hospitalized due to HF (58% vs. 40%, p=0.151) and 24 patients (35%) received appropriate therapies for ventricular tachyarrhythmias (26% vs. 46%, p=0.126).
Clinical and echocardiographic characteristics according to clinical response in patients without left ventricular reverse remodeling.
| Baseline | Clinical responders (n=38) | Non-responders (n=31) | p |
|---|---|---|---|
| Age, years, mean ± SD | 64±11 | 64±10 | 0.823 |
| Male gender, n (%) | 31 (82) | 26 (84) | 1.000 |
| Ischemic etiology, n (%) | 18 (48) | 16 (52) | 0.811 |
| NYHA class, mean ± SD | 2.95±0.40 | 2.74±0.51 | 0.074 |
| NYHA class ≥III, n (%) | 34 (90) | 25 (81) | 0.327 |
| BMI, kg/m2, mean ± SD | 26±4 | 27±4 | 0.151 |
| Obesity, n (%) | 5 (13) | 6 (19) | 0.525 |
| Diabetes, n (%) | 15 (40) | 8 (26) | 0.307 |
| History of smoking, n (%) | 16 (42) | 17 (54) | 0.456 |
| COPD, n (%) | 7 (18) | 9 (29) | 0.379 |
| AF, n (%) | 20 (53) | 16 (52) | 1.000 |
| CrCl, ml/min/1.73 m2, mean ± SD | 72±30 | 67±30 | 0.317 |
| CrCl <60 ml/min/1.73 m2, n (%) | 14 (37) | 18 (58) | 0.094 |
| Previous heart surgery, n (%) | 9 (24) | 12 (39) | 0.199 |
| LBBB, n (%) | 29 (76) | 21 (68) | 0.589 |
| QRS duration, ms, mean ± SD | 158±30 | 167±35 | 0.398 |
| QRS duration >150 ms, n (%) | 25 (66) | 22 (70) | 0.794 |
| LVEDD, mm, mean ± SD | 76±9 | 77±9 | 0.792 |
| LVESD, mm, mean ± SD | 62±9 | 63±10 | 0.912 |
| LVEF, %, mean ± SD | 23±6 | 25±6 | 0.401 |
| MR grade 3+ or 4+, n (%) | 10 (26) | 8 (26) | 1.000 |
| CRT optimization, n (%) | 10 (26) | 8 (26) | 1.000 |
| Follow-up, months, mean ± SD | 40±23 | 30±18 | 0.051 |
| Appropriate therapies, n (%) | 17 (46) | 8 (26) | 0.126 |
| All-cause mortality, n (%) | 7 (18) | 18 (58) | 0.001 |
| Cardiac death, n (%) | 3 (8) | 13 (42) | 0.001 |
| Hospitalization for HF, n (%) | 15 (40) | 18 (58) | 0.151 |
| All-cause mortality and/or hospitalization for HF, n (%) | 15 (40) | 22 (71) | 0.015 |
AF: atrial fibrillation; Appropriate therapies: antitachycardia pacing and/or shock; BMI: body mass index; COPD: chronic obstructive pulmonary disease; CrCl: creatinine clearance; CRT: cardiac resynchronization therapy; HF: heart failure; LBBB: left bundle branch block; LVEDD: left ventricular end-systolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-diastolic diameter; MR: mitral regurgitation; NYHA: New York Heart Association.
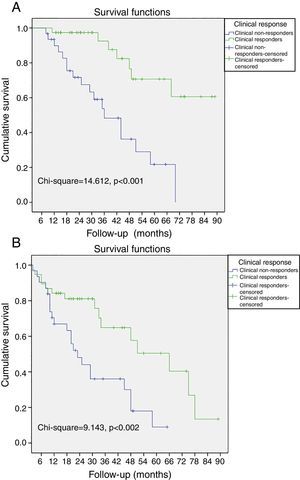
Non-responders to CRT among patients without LVRR was significantly associated with all-cause mortality, cardiac death and the combined endpoint (all-cause mortality and hospitalization due to HF). When the population was classified according to clinical response to CRT, a cumulative 3%, 4%, and 10% of the patients with improvement of ≥1 NYHA functional class died at 12-, 24-, and 36-month follow-up, respectively. In contrast, 10%, 29%, and 52% of the patients without improvement of ≥1 NYHA functional class died during the same period, respectively (log-rank p<0.001, Figure 1A). Also, a cumulative 11%, 18%, and 38% of the patients with improvement of ≥1 NYHA functional class had the combined endpoint by 12-, 24-, and 36-month follow-up, respectively, and 32%, 54%, and 67% of the patients without improvement of ≥1 NYHA functional class had the combined endpoint during the same period (log-rank p=0.002, Figure 1B).
Kaplan-Meier curves of all-cause mortality and hospitalizations due to heart failure. (A) The probability of all-cause mortality differed significantly between clinical responders and non-responders; (B) the probability of all-cause mortality and hospitalizations due to heart failure differed significantly between clinical responders and non-responders.
To determine whether clinical response in patients without LVRR was an independent predictor of all-cause mortality during follow-up, univariate predictors with a p-value <0.05 were entered into a Cox proportional hazards model as covariates (Table 3). On multivariate analysis, clinical response (hazard ratio [HR]: 0.120; 95% CI: 0.039-0.366; p<0.001) was independently associated with better survival. Reduced LVEF at baseline (HR: 4.768; 95% CI: 1.487-15.293; p=0.009), significant mitral regurgitation (grade >2+) post-CRT (HR: 2.863; 95% CI: 1.139-7.196; p=0.025) and hospitalization due to HF within 12 months of follow-up (HR: 2.459; 95% CI: 1.030-5.870; p=0.043) were associated with higher all-cause mortality.
Cox univariate and multivariate regression analysis for all-cause mortality.
| Dependent variable: all-cause mortality | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age, years | 1.035 (1.007-1.080) | 0.043 | - | - |
| Renal dysfunction | 3.861 (1.603-9.299) | 0.003 | - | - |
| LVEF <20% | 2.046 (1.056-4.681) | 0.029 | 4.768 (1.487-15.293) | 0.009 |
| MR post-CRT, grade 3+ or 4+ | 2.081 (1.105-4.664) | 0.041 | 2.863 (1.139-7.196) | 0.025 |
| RV dysfunction | 3.117(1.199-8.101) | 0.020 | - | - |
| Clinical response | 0.205 (0.084-0.501) | 0.001 | 0.120 (0.039-0.366) | <0.001 |
| Hospitalization for HF within 12 months of follow-up | 3.102 (1.405-6.852) | 0.005 | 2.459 (1.030-5.870) | 0.043 |
CI: confidence interval; CRT: cardiac resynchronization therapy; HF: heart failure; HR: hazard ratio; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; RV: right ventricular.
Renal dysfunction: glomerular filtration rate <60 ml/min/1.73 m2.
RV dysfunction: tricuspid annular plane systolic excursion <16 mm or velocity of tricuspid annular systolic motion by tissue Doppler imaging <9.5 cm/s.
As expected, the main cause of mortality was cardiac death (16 out of 25 patients). Interestingly, non-responders died more frequently for cardiovascular reasons compared with responders, although this was not statistically significant (13 [72%] vs. 3 [43%], p=0.205).
DiscussionThe major findings of the present study, which focused on the prognostic impact of clinical response to CRT in patients without LVRR six months after CRT, are: first, the majority of patients (61%) presented evidence of LVRR; second, the overall clinical response rate was 75%; third, patients with LVRR had higher survival rates and less hospitalizations due to HF; fourth, among patients without LVRR, 55% presented long-term improvement of ≥1 NYHA functional class; fifth, clinical response was the strongest independent predictor of survival in patients without LVRR.
Several studies have confirmed the favorable impact on mortality and morbidity of CRT in mid- to long-term follow-up.6–12 In our cohort, patients with LVRR showed better clinical outcomes, with higher survival rates (80% vs. 64%, p=0.023) and less hospitalizations due to HF (11% vs. 48%, p<0.001). This survival benefit may be related to the occurrence and extent of LVRR in mid-term follow-up, rather than to improvement in NYHA functional class, according to other authors.12–16,18–20 Results of the REVERSE, MADIT-CRT, and RAFT trials showed that reduction in HF readmissions and improvement in long-term survival appear to be related to LVRR and improvement of left ventricular performance rather than improvements in NYHA functional class, since the patients included were asymptomatic or only mildly symptomatic.12,18,19 Yu et al.13 showed in 141 HF patients that reductions of ≥10% in LVESV were independently associated with mid-term outcome after CRT and that improvement in clinical parameters was not predictive of long-term survival. In a retrospective and non-randomized study, Bertini et al.20 concluded that reduction in LVESV in mid-term follow-up was a better predictor of long-term survival than improvement in clinical status.
According to these data, LVRR should be a more suitable surrogate to assess the efficacy of CRT than subjective clinical parameters. Unfortunately, there are a significant proportion of non-responders to CRT, ranging between 17 and 46%, depending on the criteria used.21 In our study, 39% of patients did not present LVRR. The factors mainly identified as contributors to lack of response to CRT are ischemic etiology, shorter QRS duration and less baseline mechanical dyssynchrony.22–24 We also found that non-ischemic cardiomyopathy (74% vs. 51%, p=0.002) and LBBB (89% vs. 72%, p=0.007) were associated with LVRR. QRS duration was not found to be statistically associated with LVRR, probably due to the size of our study population. Female gender was associated with LVRR (39% vs. 17%, p=0.003), in agreement with previous studies.25,26
In the present study, 75% of patients referred for CRT presented a clinical response in long-term follow-up. This higher rate of clinical response compared to LVRR is in line with previous studies, in which clinical responders range between 61% and 77%,23,27–29 with a mean of 66.9%.30 Although improvement in NYHA functional class and LVRR are connected, the concordance between the two response types is not perfect.24 In the present study, 28% of patients (38 out of 134) with clinical improvement did not have LVRR. Previous studies found similar results, with a rate of disagreement of around 28%.25,29–32 The follow-up profile in the presence of clinical improvement without LVRR is not well known. It has been questioned whether this change is due to a placebo effect or should also be considered a positive response to CRT. Patients with improvement of ≥1 NYHA class among those without LVRR at six months after CRT showed lower all-cause mortality (18% vs. 58%, p=0.001). Also, on multivariate analysis, clinical response was independently associated with better survival (HR: 0.120, 95% CI: 0.039-0.366, p<0.001).
These conflicting findings, which contrast with previously reported results, should be interpreted with caution because of the retrospective nature of the analysis and the size of the study population. Despite this, our results show some similarities with published data. In a study of 174 HF patients, Kronborg et al.33 found that clinical response to CRT was an independent predictor of mortality in very long-term follow-up (HR: 3.02, 95% CI: 1.71-5.38, p<0.001). Improvement in functional class was addressed by another study with a different methodology, which showed that self-assessed functional class two months after CRT was a strong predictor of long-term survival (HR: 0.59, 95% CI: 0.40-0.87, p<0.007).34 These studies show that symptomatic response after CRT is associated with better survival and are consistent with data previously described by Molhoek et al.35 and Cha et al.36 Moreover, the low annual mortality in our population (≈8%) confirms the synergistic interaction between administration of neurohumoral antagonists, CRT, and cardioverter-defibrillator implantation in a daily practice setting.
Lower LVEF at baseline was also related to mortality in our study. This is supported by a large multicenter prospective study.37 In addition, significant mitral regurgitation (grade >2+) post-CRT was found to be independently associated with all-cause mortality, as also described in the Cardiac Resynchronization in Heart Failure Trial38 and by van Bommel et al.39 Another clinical parameter with impact on mortality was hospitalization due to HF during the first year of follow-up. The importance of HF admissions after CRT was also shown by Bertini et al.,20 and data from the MADIT-CRT trial revealed an association between future events and HF admission during the 12 months before CRT implantation.40
Finally, these data indicate that lack of clinical improvement in patients without LVRR after CRT was a strong marker of worse prognosis. Its effect was independent of age, renal function and right ventricular dysfunction.
LimitationsThis was a retrospective, single-center, non-randomized and non-controlled study, and this should be taken into consideration when interpreting the results. Notwithstanding, this represents a real-life group of patients followed in a referral center. Secondly, clinical response was based on improvement in NYHA class and did not include assessment of functional capacity or quality of life scores. Thirdly, clinical and echocardiographic response was considered at six-month follow-up, but some patients may have had late LVRR.41 Fourthly, there will always be a certain degree of intra- and inter-observer variability in analysis of echocardiographic parameters, whatever the operator's expertise. Fifthly, other parameters such as position of the left ventricular lead, device programming, presence of rhythm abnormalities, extent of myocardial scar and brain natriuretic peptide levels were not addressed in this study. Finally, due to the small number of patients included in the study, its findings regarding predictors of all-cause mortality need confirmation in large-scale prospective studies.
ConclusionsHF patients treated with CRT without LVRR at mid-term follow-up had a worse long-term prognosis than patients with LVRR, with a high rate of hospitalizations due to HF and all-cause mortality. However, among these patients, clinical response appeared to be independently associated with better survival. Further studies are needed to clarify the prognostic impact of a sustained clinical response in the absence of LVRR following CRT.
Conflicts of interestThe authors have no conflicts of interest to declare.