Transcatheter aortic valve implantation (TAVI) is an alternative to surgical aortic valve replacement in patients with severe aortic stenosis (AS) and unacceptably high surgical risk.
MethodsWe present our first two years’ experience with TAVI. A total of 76 AS patients were evaluated for TAVI and 23 of them underwent a TAVI procedure. These patients had a mean EuroSCORE of 22.4% and a mean age of 81.5 years, and were prospectively followed for a mean of 12.9±11 months.
ResultsThe percutaneous aortic valve was successfully implanted in 100% of the patients. Mortality at 30 days was 4%. The most common complications were access site-related bleeding and transfusion (22%), followed by new permanent pacemaker implantation (9%). After a mean follow-up of 12.9 months, survival was 87%. In a maximum follow-up of 30 months there were no cases of prosthesis dysfunction or cardiovascular death.
ConclusionsTwo years after the introduction of a TAVI program in our center, the procedure has established itself as a safe and effective alternative for patients with severe AS and unacceptably high surgical risk.
A implantação da válvula aórtica transcateter (TAVI) é uma alternativa à substituição valvular aórtica cirúrgica convencional para doentes com estenose aórtica (EA) grave e risco cirúrgico inaceitável.
MétodosApresentamos a experiência com TAVI no nosso centro durante os primeiros anos, desde o seu início. De 76 doentes com EA avaliados para eventual TAVI, realizou-se o procedimento em 23 dos mesmos, que apresentavam um Euroscore médio de 22,4% e uma idade média de 81,5 anos. Estes 23 doentes foram seguidos de modo prospectivo durante 12,9±11 meses.
ResultadosA prótese foi implantada com êxito em todos os doentes. A mortalidade aos 30 dias foi de 4%. As complicações mais frequentes foram as vasculares e a necessidade de transfusão (22%) seguida de colocação de pace-maker definitivo (9%). Após um follow-up médio de 30 meses não se registou nenhum caso de disfunção protésica nem de morte cardiovascular.
ConclusõesDois anos após o início de um programa de TAVI no nosso centro, a TAVI evidencia-se como uma alternativa eficaz para doentes com EA grave inoperáveis por risco cirúrgico elevado.
The course of aortic stenosis (AS) is slow and asymptomatic for most of its natural history, but progresses rapidly in its more advanced stages. Mortality two years after symptom onset is 50%, and when there are symptoms of heart failure, it is 50% at one year.1 AS is the most common isolated valve disease, accounting for around 40% of all diagnoses, and is the most frequent indication for valve surgery. Over the age of 75 years, the prevalence of severe AS can reach 4.6% in the general population, a figure that is expected to rise with aging populations.2
The standard treatment for severe symptomatic AS is valve replacement surgery, which has been shown to improve survival.3,4 However, in daily clinical practice, around a third of AS patients who in principle are indicated for surgical replacement are rejected because of high surgical risk, mainly due to the frequent association of advanced age and comorbidities.5,6 An alternative treatment for patients with severe symptomatic AS who are refused for surgery has recently been developed, which consists of percutaneous implantation of a prosthetic valve mounted on a metal stent, known as transcatheter aortic valve implantation (TAVI). This procedure avoids the morbidity and mortality associated with sternotomy and extracorporeal circulation, and can be performed by apical ventriculotomy or a transarterial approach (usually transfemoral).7
We present our center's experience with TAVI during the first two years since its introduction.
MethodsIn 2008 a TAVI program was introduced using a transfemoral approach.8 A total of 23 Edwards SAPIEN prosthetic valves (Edwards Lifesciences, USA) have been implanted to date and these patients have been followed prospectively through outpatient appointments and serial echocardiograms. The aim of this study is to describe patient selection, immediate results and clinical course in these patients.
Clinical data for this observational, prospective cohort study were collected during appointments before the procedure or during hospital stay. After discharge, follow-up echocardiograms were performed at one month, six months and one year. Clinical follow-up was by outpatient consultations and all patients were contacted.
Patient selectionAll patients with severe symptomatic degenerative AS rejected for surgical replacement were assessed for TAVI by a femoral approach over a period of two years. Our center's assessment protocol has been described previously.9 Severe AS was defined as an aortic valve area calculated by planimetry or the continuity equation of <1cm2 or <0.6cm2/m2 indexed to body surface area by echocardiography, as recommended in the ESC guidelines.4 Patients were assessed by a team of clinical and interventional cardiologists, cardiac surgeons and anesthesiologists who evaluated surgical risk, with the help of standard risk scores: EuroSCORE and the Society of Thoracic Surgeons (STS) Predicted Risk of Mortality.10,11 Patients rejected for surgery (those with logistic EuroSCORE >20% and/or STS >10%) were assessed for transfemoral TAVI, all undergoing coronary catheterization and femoral angiography, transesophageal echocardiography to measure aortic valve annulus diameter, and high-resolution CT angiography of the iliofemoral axis.
Patients with bicuspid aortic valve, valve annulus <18mm or >25mm, left ventricular ejection fraction (LVEF) <20%, myocardial infarction in the previous 30 days, or stroke or transient ischemic attack in the previous 6 months, were excluded. Those with significant coronary disease were scheduled for percutaneous coronary intervention (PCI) and TAVI was scheduled for 30 days after PCI. Also excluded were patients with excessive tortuosity or calcification, and those with a minimum iliofemoral axis diameter of <7mm with the first-generation RetroFlex® catheter, and from May 2010 onwards, <6mm with the second-generation NovaFlex® catheter. Although severe septal hypertrophy has been considered an exclusion criterion, due to the risk of prosthesis embolization during implantation, in our center the implantation procedure is slightly modified in these patients.12
In the 30-month study period (April 2008–October 2010), a total of 76 patients were evaluated, of whom 44 (57.9%) were rejected for TAVI, 25 (32.9%) were accepted, 5 (6.6%) died during the evaluation period, and 2 (2.6%) are currently on the waiting list for the first transapical implantation in our center. Of the 44 patients rejected, the reason was poor iliofemoral access in 17 (38.6%), comorbidities that significantly limited the patient's life expectancy or quality of life in 8 (18.2%), non-severe or asymptomatic AS in 7 (15.9%), refusal by the patient or the patient's family in 5 (11.4%), and severe ventricular dysfunction (LVEF<20%) in 4 (9.1%); four patients (9.1%) were referred again for surgical valve replacement after re-evaluation showed the surgical risk to be acceptable. Of the 25 patients accepted for TAVI, implantation proved impossible in 2 (8%) due to vascular access complications. The remaining 23 patients (92%) constitute the study population.
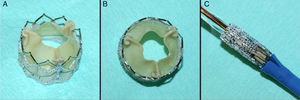
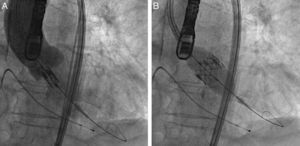
ProcedureThe procedure was performed under general anesthesia in a sterile environment in the hemodynamic laboratory, with patients being extubated in the same room in most cases. The Edwards SAPIEN aortic prosthesis consists of a trileaflet bovine pericardial prosthesis mounted on a balloon-expandable metal stent (Fig. 1). Two sizes are currently available: 23mm (for a valve annulus of 18–21.5mm) and 26mm (for a valve annulus of 21.5–25mm). A temporary pacemaker lead is positioned via the femoral vein in the right ventricle, and balloon aortic valvuloplasty is performed under rapid ventricular pacing (∼200bpm) following the standard technique. A retrograde approach is used as described previously.13 The sheath is introduced by sequential dilation of the access site from the femoral artery to the infrarenal aorta, and through this the balloon-mounted prosthesis is inserted. The system is advanced through the native valve and the balloon is inflated, again under rapid pacing, to expand the stent and implant it in the native valve annulus, compressing the valve leaflets (Fig. 2). The procedure is monitored by angiography and three-dimensional transesophageal echocardiography. The antiplatelet therapy prescribed for most patients was aspirin 100mg/day and clopidogrel 75mg/day for three months.
The variables analyzed in this study are those recommended by consensus documents and assessed in other TAVI registries.14,15 The data were stored in a specially designed database and analyzed using SPSS 15.0 (Chicago, Illinois, USA). Categorical variables are expressed as percentages and continuous variables as means±standard deviation. Mortality during follow-up is shown by Kaplan–Meier survival curve analysis.
ResultsStudy populationTAVI was performed in a total of 23 patients, whose baseline characteristics are summarized in Table 1. Mean age was 8.15±6 years (68–90). The most common comorbidity was ischemic heart disease, in 13 patients (56.5%), 10 of whom had undergone revascularization prior to aortic valve implantation (four by coronary artery bypass grafting and six by PCI). Ten (43.5%) had chronic renal failure, of whom two (8.7%) were undergoing dialysis.
Characteristics of the study population.
| n=23 | % | |
| Age (years) (mean) | 81.5±6 | |
| Male | 14 | 60.1 |
| Comorbidities | ||
| Chronic pulmonary disease | 7 | 30.4 |
| Chronic renal failure | 10 | 43.5 |
| Peripheral arterial disease | 2 | 9.6 |
| Cerebrovascular disease | 2 | 9.6 |
| Ischemic heart disease | 13 | 56.5 |
| Previous PCI | 6 | 26.1 |
| Previous PMV | 1 | 4.4 |
| Previous CABG | 4 | 17.4 |
| NYHA class III/IV | 13 | 56.5 |
| Atrial fibrillation | 11 | 47.8 |
| Echocardiographic data | ||
| Mean aortic valve area | 0.69±0.2 | |
| Mean aortic gradient | 42±11 | |
| LVEF <50% | 4 | 17.4 |
| Aortic regurgitation grade ≥2 | 5 | 21.7 |
| Aortic regurgitation grade ≥2 | 7 | 30.4 |
| Mean PASP | 52.2±9 | |
| EuroSCORE (mean) | 22.4±13 | |
CABG: coronary artery bypass grafting; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; PASP: pulmonary artery systolic pressure; PCI: percutaneous coronary intervention; PMV: percutaneous mitral valvuloplasty.
The etiology of AS was degenerative in all patients except one (rheumatic valve disease). Of those with coexisting valve disease, 3 (13%) had moderate mitral stenosis, 7 (30%) had moderate mitral regurgitation and 7 (30%) had moderate or severe tricuspid regurgitation. Severe mitral regurgitation was considered an exclusion criterion, but one such patient first underwent percutaneous mitral valvuloplasty followed by TAVI.
Procedural resultsTable 2 shows the procedural results. The valve was successfully implanted in all cases. The hemodynamics of the newly implanted valve was excellent, a valve area of over 2cm2 being achieved in all cases. Around half of patients (n=12; 52.2%) were implanted using the first-generation system (RetroFlex®, 22 and 24 F), and the rest (n=11; 47.8%), using the second-generation system (NovaFlex®, 18 and 19 F). The introduction of the NovaFlex® system in May 2010 led to a significant reduction in the number of patients rejected for TAVI because of insufficient iliofemoral diameter, as well as fewer vascular access complications: four out of 12 (33%) patients with the RetroFlex® vs. none out of 11 with the NovaFlex® (chi-square, p=0.015).
Procedural data.
| n=23 | % | |
| Valve implanted, mm | ||
| 23 | 16 | 69.6 |
| 26 | 7 | 30.4 |
| Procedural success | 23 | 100 |
| Urgent cardiac surgery | 0 | 0 |
| Post-procedural echocardiographic data | ||
| Mean aortic valve area (cm2) | 2.7±0.6 | 2.2 |
| Mean aortic gradient (mmHg) | 5.3±1 | 9.2 |
| Moderate prosthetic leak | 1 | 4.4 |
| Severe prosthetic leak | 0 | 0.0 |
| Mean hospital stay (days) | 11.9±6 | 11.9 |
Complications during and after the procedure are shown in Table 3. There were no deaths in the hemodynamic laboratory (intraprocedural mortality 0%). One patient died on the third day after implantation due to cardiac tamponade; the autopsy revealed perforation of the right ventricle by the pacemaker lead.16 Mortality at 30 days was thus 4.4%.
Complications during hospital stay, from discharge to 30 days, and 2–12 months after implantation (n=23).
| In-hospital | 30 daysa | 1 year | Total | |
| Any complication | 8 (34.8%) | 1 (4.4%) | 5 (22.7%) | 14 (61%) |
| Death | 1 (4.4%) | 0 | 2 (8.7%) | 3 (13%) |
| Implant failure | 0 | 0 | 0 | 0 |
| Stroke | 0 | 0 | 1 (4.4%) | 1 (4.4%) |
| Renal failure requiring HD | 0 | 0 | 0 | 0 |
| Prosthesis embolization | 0 | 0 | 0 | 0 |
| MI/coronary obstruction | 0 | 0 | 0 | 0 |
| Permanent pacemaker | 2 (8.7%) | 0 | 0 | 2 (8.7%) |
| Vascular complications | ||||
| Major | 1 (4.4%) | 0 | 0 | 1 (4.4%) |
| Minor | 5 (22.7%) | 1 (4.4%) | 0 | 5 (22.7%) |
| Transfusion | 5 (22.7%) | 0 | 0 | 5 (22.7%) |
| Rehospitalization | NA | 0 | 2 (8.7%) | 2 (8.7%) |
HD: hemodialysis; MI: myocardial infarction.
The following major complications occurred: laceration of the femoral artery requiring immediate surgical repair in one case, need for transfusion in five cases (22%), and need for permanent pacemaker implantation in two cases (9%). The latter were due to damage to the atrioventricular node and His bundle by prosthesis expansion.16 There were no cases of significant prosthesis dysfunction.
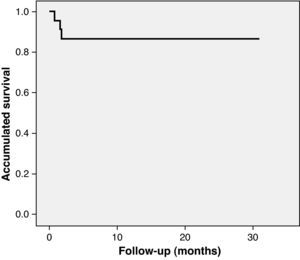
Long-term follow-upClinical follow-up was achieved in all patients. Mean follow-up was 12.9±11 months. The first patient to undergo TAVI in our center has now been followed for 30 months and is alive, with good prosthesis function. Between the 30th day and the end of follow-up two more patients died (8.7%), both between the first and second month after the intervention and neither due to cardiac cause (one from pneumonia and the other following general deterioration and multiple organ failure). Accumulated mortality at the end of follow-up was 13%. As shown by Kaplan–Meier survival analysis, all deaths occurred in the first three months, the survival curve being flat thereafter (Fig. 3). With regard to major adverse cardiovascular events (rehospitalization for prosthesis dysfunction, heart failure, stroke or myocardial infarction), two patients were rehospitalized, one for heart failure at two months (with normal valve function) and the other at three months for stroke. Survival free of major events (death or cardiovascular rehospitalization) was 91.3% at one month and 78.3% at one year. No cases of prosthesis dysfunction were recorded during follow-up.
DiscussionImpact of aortic stenosisDegenerative AS is the most common valve disease, and its prevalence is projected to increase in the coming years due to aging populations.17 This has implications not only for those specializing in cardiovascular disease; AS is increasingly diagnosed and treated by other medical specialties, including internal medicine, geriatrics and intensive care.
Transcatheter aortic valve implantationValve replacement surgery is an effective and safe treatment that changes the natural history of AS, but in certain circumstances, especially at advanced ages, it can carry an unacceptably high surgical risk.3,4 The recent development of transcatheter aortic valve implantation has provided an alternative treatment for such patients that is being used in an increasing number of centers. In this article we present the results of this technique in the first two years since its introduction in our center.
In our series, 30-day mortality was 4.4%, an excellent result considering that the surgical mortality predicted by the EuroSCORE in this population was 22%. These results are comparable to those of other published series; in the SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) Registry, with 463 patients treated by a transfemoral approach, initial implantation success was 95.2%, and 30-day mortality was 6.3%.15 In the Placement of Aortic Transcatheter Valve (PARTNER) trial, 358 patients with severe symptomatic AS refused for surgery were randomized to TAVI or standard therapy (medical and/or balloon aortic valvuloplasty). One-year mortality was 30.7% in the TAVI group and 50.7% in the standard therapy group (p<0.001), an absolute reduction of 20% and a relative reduction of 40%.13
The rate of complete atrioventricular block requiring permanent pacemaker implantation in our series (9%) was comparable to that of other series and well below that of studies on the other currently available transcatheter-implantable aortic valve prosthesis (CoreValve, Medtronic, USA).18
Long-term follow-up in our population has shown excellent results, with one-year survival of 87% and no deaths from cardiac cause or complications associated with the prosthetic valve such as prosthesis dysfunction and endocarditis. One-year survival in the SOURCE registry was 81.1%.19
Establishing a TAVI program: practical considerationsAlthough TAVI is actually performed by interventional cardiologists, a variety of other medical personnel are involved throughout the process, from diagnosis to discharge and follow-up. Degenerative AS is diagnosed and managed not only in cardiology departments but also often by those working in primary care, internal medicine, geriatrics, intensive care or other environments, who work with cardiologists, cardiac surgeons and anesthesiologists, in consultation with patients and their families to decide whether to proceed with valve replacement surgery, based on the patient's clinical condition and surgical risk. As our study demonstrates, TAVI is a new therapeutic option for patients rejected for surgery due to high surgical risk that provides effective treatment for those who previously had no possibility of valve replacement.
The technical aspects of the procedure require not only interventional cardiologists, but also many other medical personnel specifically trained in the technique, including cardiologists specializing in transesophageal echocardiography, vascular surgeons, interventional radiologists and cardiovascular anesthesiologists. This range of specialties reflects the increasingly multidisciplinary nature of modern medicine.
One important aspect is the need for a rapid selection process, since short-term mortality in these patients is significant.1 In our series, five patients (6.6% of the total) died during evaluation or while on the waiting list, less than three months after first clinical contact with our group. These figures reveal the terminal condition of many AS patients.20
Further technical improvements in valve prostheses, and accumulated experience, can be expected to reduce mortality and risk of procedure-related complications in the future. New valve technology is at various stages of development, including changes in valve design and release mechanisms, which will lead to the technique becoming more widespread. In this context, the results of the A cohort of the PARTNER trial are eagerly awaited: patients accepted for surgical valve replacement but with relatively high risk are randomized to either surgery or TAVI, the first time transcatheter replacement has been compared directly with the standard treatment. The results of this trial may well lead to the indication for TAVI being extended to patients accepted for surgery but with relatively high surgical risk. It is expected that a risk score for TAVI will soon be developed that will predict mortality and complications, so as to improve the information available to patients and their families and to facilitate comparison with surgical replacement. Studies will also need to be performed on the cost-effectiveness of TAVI compared to standard therapy and surgery, since at the moment the technique is costly.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful to the Departments of Cardiovascular Anesthesia, Vascular Radiology, Vascular Surgery, Cardiac Surgery, and the Cardiac Care Unit of Hospital La Paz, Madrid, and to Dr. David Filgueiras for his assistance with patient selection. Pablo Salinas received a Cordis grant from the Spanish Society of Cardiology for training in interventional hemodynamics and interventional cardiology.
Please cite this article as: Salinas P. Implantação percutânea de próteses valvulares aórticas: resultados de uma nova opção terapêutica na estenose aórtica com alto risco cirúrgico. Rev Port Cardiol. 2011. doi:10.1016/j.repc.2011.12.008.