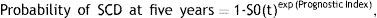Previous studies have demonstrated the predictive value of the neutrophil-to-lymphocyte ratio (NLR) in many cardiovascular disorders. The aim of this study was to assess whether NLR is associated with echocardiographic or electrocardiographic parameters, or with predicted five-year risk of sudden cardiac death (SCD), in patients with hypertrophic cardiomyopathy (HCM).
MethodsThis prospective observational study included 74 controls and 97 HCM patients. Three years of follow-up results for HCM patients were evaluated.
ResultsNLR was significantly higher in patients with fragmented QRS, ventricular tachycardia, and presyncope than in those without (p=0.031, 0.030, and 0.020, respectively). NLR was significantly higher in patients whose predicted five-year risk of SCD was more than 6% and whose corrected QT interval was greater than 440 ms (p=0.022 and 0.001, respectively). It was also significantly higher in patients whose left ventricular ejection fraction (LVEF) was <60% than in those with LVEF >60% (p=0.017).
ConclusionNLR was significantly higher in patients with HCM compared to the control group. A high NLR is associated with a higher five-year risk of SCD in patients with HCM.
Estudos prévios demonstraram o valor preditivo da relação neutrófilos/linfócitos (RNL) em muitas alterações cardiovasculares. O objetivo deste estudo foi avaliar se a RNL está associada a parâmetros ecocardiográficos ou eletrocardiográficos, ou com o score de risco de morte súbita cardíaca (MSC) a cinco anos em doentes com miocardiopatia hipertrófica (MCH).
MétodosEste estudo prospetivo observacional incluiu 74 controlos e 97 doentes com MCH. Foram avaliados os resultados dos doentes com MCH ao longo de três anos de seguimento.
ResultadosO valor da RNL foi significativamente superior nos doentes com QRS fragmentado, com taquicardia ventricular e com pré-síncope do que nos que não revelaram esses sinais (valores p: 0,031, 0,030, 0,020, respetivamente). O valor RNL foi estatística e significativamente superior nos doentes com risco de MSC previsível a cinco anos superior a 6%, e com um intervalo QT corrigido superior a 440ms (valores p: 0,022, 0,001, respetivamente). O valor da RNL foi significativamente superior nos doentes com fração de ejeção (FE)<60% do que nos doentes com FE>60% (valor p=0,017).
ConclusãoA RNL foi significativamente superior nos doentes com MCH quando comparada com o grupo controlo. Uma RNL alta está associada a um score de risco de MSC elevado aos cinco anos em doentes com MCH.
Hypertrophic cardiomyopathy (HCM) is a relatively common genetic cardiac disorder that can appear at any age. It affects men and women equally and is a leading cause of sudden cardiac death (SCD), especially in young adults.1 This is the most devastating result of its natural history. Although there have been numerous studies of HCM patients, no risk stratification strategy will ever be able to predict SCD with absolute certainty.
The neutrophil-to-lymphocyte ratio (NLR) is an easily accessible and widely available novel hematological marker of oxidative stress damage. It also serves as a good prognostic marker and has been studied in patients with infectious diseases, as well as in cases of oncological, hematological, immunological, and cardiac disorders, using a complete blood count, the most commonly performed test in hospitals. Previous research has revealed that it has significant prognostic value, in addition to various positive correlations. The predictive value of NLR in peripheral arterial disease and calcific aortic stenosis, as well as in prognostication of the presence, severity, and extent of coronary artery disease, heart failure, and many other cardiovascular disorders has already been demonstrated.2,3 A high NLR is associated with poor prognosis in the majority of studies. Although the relationship between NLR and almost all cardiovascular disorders has been investigated, the importance of NLR in HCM remains unclear. To the best of our knowledge, few studies have been conducted on the prognostic significance of the correlation between NLR and HCM.
In this study, we aimed to assess whether the presence of high NLR is associated with poor prognosis in HCM and whether NLR is associated with echocardiographic parameters, cardiac arrhythmia, or predicted five-year risk of SCD.
MethodsPatient populationThis prospective observational study included a consecutive sample of 97 patients with HCM and 74 age- and gender-matched controls without HCM who presented to Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Center, Training and Research Hospital, and to the School of Medicine at Bezmialem Vakif University, between December 2012 and November 2015. Three years of follow-up results of patients with HCM were evaluated. The study was approved by the ethics committee of the School of Medicine at Bezmialem Vakif University, and all participants gave their written informed consent.
The study inclusion criteria were age >17 years and echocardiography or cardiac magnetic resonance imaging (CMRI) revealing HCM, defined as left ventricular (LV) wall thickness of at least 15 mm in one or more LV myocardial segments.4 In those with lesser degrees of wall thickening (13-14 mm) with a high possibility of HCM, we evaluated other factors, including family history, positive gene mutations, and abnormalities on the electrocardiogram (ECG).
Patients with hypertension (n=8), renal failure (n=2), history of myocardial infarction (n=1), aortic valve stenosis (n=1), and active inflammation or chronic inflammatory diseases (n=2), were excluded from the study, resulting in a final population of 97 patients.
ElectrocardiographyA resting 12-lead surface ECG was obtained from all patients while in the supine position. The ECG recordings were made on a Nihon Kohden Cardiofax S ECG-1250K (filter range 0.5-150 Hz, AC filter 60 Hz, at a speed of 25 mm/s and an amplitude of 10 mm/mV) at the first visit. Cardiac rhythm, heart rate, the presence of fragmented QRS (fQRS) and QRS, QT, and corrected QT (QTc) interval were determined.
Fragmented QRSThe presence or absence of fQRS on the ECGs was assessed by two independent readers blinded to both cardiac markers and clinical outcomes. Interobserver agreement with regard to detection of fQRS was 96.8% (j=0.93) between the two readers and intraobserver agreement was 98.9% (j=0.97). If the observers could not reach an agreement, a third independent observer was included in the decision-making, and an agreement between two of the three observers was accepted as the final decision. fQRS was defined as the presence of an extra R wave (R1) on a 12-lead ECG, notching on an R wave, notching on an S wave, or the presence of more than one R1 wave in two adjacent leads corresponding to the territory of one of the major coronary arteries.5 Complete and incomplete bundle branch block and paced rhythm were excluded from the definition of fQRS.
EchocardiographyOn admission to the hospital, participants underwent transthoracic echocardiographic studies using a Vivid S5 and a 3S-RS probe (GE Healthcare) with a 1.7/3.4 MHz phased-array transducer. All echocardiographic parameters were measured off-line based on an average of three cardiac cycles. LV ejection fraction (LVEF) was calculated using the biplane Simpson method.6 LV wall thickness (interventricular septum, posterior wall), LV end-diastolic diameter (LVEDD), and LV end-systolic diameter (LVESD) were measured in parasternal long-axis view. The LV outflow tract gradient was measured in apical 5-chamber view. In addition, LVEDD, LVESD, left atrial (LA) diameter, LA volume, LA volume index (LAVI), LV mass (LVM), LV mass index (LVMI), mitral valve regurgitation and systolic anterior motion of the mitral valve, relative wall thickness index (RWTI), and LV diastolic dysfunction were assessed.
Holter ambulatory electrocardiographic monitoringHolter 12-lead 24-hour records (DMS 300-7 Holter recorder, DM Software, Stateline, NV, USA) were obtained from all patients and analyzed automatically using CardioScan 12.0 (DM Software). The recordings were analyzed for rhythm, premature atrial contractions, supraventricular tachycardia (VT), paroxysmal atrial fibrillation, premature ventricular contractions, nonsustained and/or sustained VT, and atrioventricular block with pauses.
Assessment of five-year risk of sudden cardiac deathAn individual patient's five-year risk of SCD was calculated using the following equation, proposed by O’Mahony et al., based on Cox proportional hazards analysis of age, non-sustained VT, maximum LV wall thickness, family history of SCD, LA diameter, unexplained syncope, and LV outflow tract gradient:
where S0(t) is the average survival probability at time t (i.e., at five years) and the prognostic index is the sum of the products of the predictors and their coefficients.7Five-year SCD risk calculation was performed for each patient at the first visit and was repeated if there were any changes in the patient's clinical status or Holter and echocardiography results. Patients were divided into two groups based on their predicted risk, ≤6% or >6%.
Biochemical analysesPeripheral venous blood samples were obtained from all study participants during the first outpatient clinic visit and collected in K3 EDTA tubes. Hematologic indices were calculated from the complete blood count, which was performed using a XT-2000i analyzer (Sysmex Corporation of America, Long Grove, IL, USA). NIR was calculated by dividing the neutrophil count by the lymphocyte count.
Statistical analysisThe statistical analysis was performed using SPSS version 15 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean±standard deviation. Categorical variables were expressed as frequencies and percentages. Samples were compared using the Kolmogorov-Smirnov test. Because the distribution of data was parametric, Student's t tests were performed. A Pearson's correlation coefficient analysis was performed to evaluate the relationship between the two types of quantitative data. The Student's t test was used to compare data obtained from the groups, and chi-square tests were performed to compare rates between the groups. A p value of <0.05 was accepted as statistically significant.
Study endpoints and follow-upOn admission, patients’ medical history, family history of SCD, occurrence of syncope, and responses to a questionnaire on lifestyle and risk factors were recorded. Patients were regularly followed during visits to the HCM outpatient clinic at three-month intervals and any change in clinical status was noted. ECG was performed every three months and 24-hour Holter monitoring was performed at least once in all patients, and at least twice in those with more than one risk factor for SCD. Twenty-four-hour Holter monitoring or telemetry was also performed when patients had any potentially arrhythmic symptoms, including dizziness, light-headedness, palpitations, or syncope. The primary endpoint for the study was occurrence of a ventricular arrhythmic event and the secondary endpoint was occurrence of a major arrhythmic event. Follow-up for clinical endpoints was performed by telephone interview and review of outpatient and inpatient medical records. The mean follow-up period was 34.95±10.23 months.
ResultsA total of 97 patients (58 male and 39 female) with HCM comprised the patient group, and the control group consisted of 74 age- and gender-matched subjects without HCM (29 male and 45 female). The participants’ demographic and clinical characteristics are summarized in Table 1. There were no significant differences between the patient and control groups with regard to age, gender, or body mass index. Hypertension, hyperlipidemia, diabetes, smoking, and total cholesterol were not statistically different between the two groups.
Baseline and clinical characteristics of patient and control groups.
| Patient group, n (%) or mean±SD | Control group, n (%) or mean±SD | p | |
|---|---|---|---|
| No. of patients | 97 | 74 | |
| Male (n) | 58 (59.8%) | 29 (43%) | 0.053 |
| Age (years) | 47.6±15.0 | 45.0±8.9 | 0.650 |
| BMI (kg/m2) | 27.1±3.6 | 27.8±4.1 | 0.169 |
| NYHA III or IV | 25 (25%) | 0 | <0.00 |
| Diabetes | 7 (7.2%) | 11 (14.9%) | 0.133 |
| Hypertension | 5 (5.2%) | 13 (17.6%) | 0.015 |
| Hyperlipidemia | 22 (22.7%) | 14 (19%) | 0.576 |
| Total cholesterol (mg/dl) | 184.6±36 | 193±34.8 | 0.137 |
| Smoking | 21 (21.6%) | 22 (29.7%) | 0.286 |
| Medical therapy, n (%) | |||
| Beta-blockers | 89 (91.8%) | 14 (18.9%) | <0.00 |
| CCBs | 5 (5.2%) | 6 (8.1%) | 0.534 |
| Amiodarone | 4 (4.1%) | 0 | 0.134 |
| Disopyramide | 8 (8.2%) | 0 | 0.10 |
| Paroxysmal AF (n) | 3 (3.1%) | 0 | 0.259 |
| NLR | 2.0±1.3 | 1.3±0.3 | <0.001 |
| LVEF (%) | 66±7.2 | 65.2±7.7 | 0.01 |
| LVMI (g/m2) | 178±48 | 77±18 | <0.001 |
| NSVT (n) | 16 (16.5%) | 0 | <0.001 |
| LA diameter (mm) | 42±4.7 | 34.6±3.3 | <0.001 |
| Peak LVOTG (mmHg) | 40±38 | 0 | <0.001 |
| LVEDD (mm) | 43±6 | 45±3.7 | 0.41 |
| RWTI | 0.62±0.24 | 0.39±0.05 | <0.001 |
| Syncope (n) | 11 (11.3%) | 0 | 0.003 |
AF: atrial fibrillation; BMI: body mass index; CCBs: calcium channel blockers; LA: left atrial; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVOTG: left ventricular outflow gradient at rest; LVMI: left ventricular mass index; NLR: neutrophil-to-lymphocyte ratio; NSVT: nonsustained ventricular tachycardia; NYHA: New York Heart Association; RWT: relative wall thickness index.
Medical treatments administered to participants in the patient and control groups are shown in Table 1. Values of NRL, LVMI, nonsustained VT, LA diameter, peak LVOTG, RWTI, and syncope were significantly higher in the patient group than in the control group (2.0±1.3 vs. 1.3±0.3, p<0.001; 178±48 vs. 77±18 g/m2, p<0.001; 16 [16.5%] vs. 0, p<0.001; 42±4.7 vs. 34.6±3.3 mm, p<0.001; 40±38 vs. 0 mmHg; 0.62±0.24 vs. 0.39±0.05, p<0.001; and 11 [11.3%] vs. 0, p<0.003, respectively).
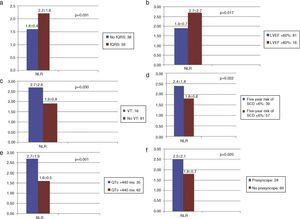
Comparisons of NRL between the groups according to fQRS, LVEF, VT, five-year SCD risk, QTc, syncope, and presyncope in patients with HCM are shown in Table 2. NLR was significantly higher in patients with fQRS, VT, and presyncope than in those without (fQRS [n=59], NLR: 2.2±1.6 vs. no fQRS [n=38], NLR: 1.6±0.4, p=0.031; VT [n=16], NLR: 2.7±2.6 vs. no VT [n=81], NLR: 1.9±0.8, p=0.030; presyncope [n=28], NLR: 2.5±2.1 vs. no presyncope [n=69], NLR: 1.8±0.7, p=0.020) (Figure 1a, c and f). NLR was significantly higher in patients whose left ventricular ejection fraction (LVEF) was <60% (LVEF ≤59.9% [n=16], NLR: 2.7±2.7 vs. LVEF >60% [n=81], NLR: 1.9±0.7, p=0.017) (Figure 1b). NLR was significantly higher in patients whose predicted five-year risk of SCD was over 6% and whose QTc interval was greater than 440 ms (predicted five-year SCD risk >6% [n=39], NLR: 2.4±1.8 vs. ≤5.9% [n=57], NLR: 1.8±0.6, p=0.022; QTc >440 ms [n=35], NLR: 2.7±1.9 vs. ≤439 ms [n=62], NLR: 1.6±0.5, p=0.001) (Figure 1d and e).
Neutrophil-to-lymphocyte ratio according to fragmented QRS, ejection fraction, ventricular tachycardia, five-year risk of sudden cardiac death, corrected QT, syncope, and presyncope in patients with hypertrophic cardiomyopathy.
| Variable | NLR | p | |
|---|---|---|---|
| fQRS | (+) (n=59) | 2.2±1.6 | 0.031 |
| (-) (n=38) | 1.6±0.4 | ||
| LVEF (%) | >60 (n=81) | 1.9±0.7 | 0.017 |
| ≤59.9 (n=16) | 2.7±2.7 | ||
| VT | (+) (n=16) | 2.7±2.6 | 0.030 |
| (-) (n=81) | 1.9±0.8 | ||
| Predicted five-year SCD risk | >%6 (n=39) | 2.4±1.8 | 0.022 |
| ≤%5.9 (n=57) | 1.8±0.6 | ||
| QTc | >440 (n=35) | 2.7±1.9 | 0.001 |
| ≤439 (n=62) | 1.6±0.5 | ||
| Syncope | (+) (n=11) | 1.7±0.9 | 0.487 |
| (-) (n=86) | 2.0±1.3 | ||
| Presyncope | (+) (n=28) | 2.5±2.1 | 0.020 |
| (-) (n=69) | 1.8±0.7 |
fQRS: fragmented QRS; HCM: hypertrophic cardiomyopathy; LVEF: left ventricular ejection fraction; NLR: neutrophil-to-lymphocyte ratio; QTc: corrected QT interval; SCD: sudden cardiac death; VT: ventricular tachycardia.
Neutrophil-to-lymphocyte ratio and (a) fragmented QRS, (b) ejection fraction, (c) ventricular tachycardia, (d) predicted five-year risk of sudden cardiac death, (e) corrected QT, and (f) presyncope in patients with hypertrophic cardiomyopathy. fQRS: fragmented QRS; LVEF: left ventricular ejection fraction; NLR: neutrophil-to-lymphocyte ratio; QTc: corrected QT interval; SCD: sudden cardiac death; VT: ventricular tachycardia.
Correlations between NLR and demographic findings, biochemical parameters, acute phase reactants, and echocardiographic results are presented in Table 3. A statistically significant correlation was found only between QTc and NLR (r=0.251, p=0.013).
Correlation between neutrophil-to-lymphocyte ratio and other variables.
| Variable (mean±SD) | NLR (mean 2.0±1.3) | ||
|---|---|---|---|
| r | p | ||
| Galectin 3 | 7138.9±2211.6 | 0.174 | 0.180 |
| Predicted five-year SCD risk | 6.0±4.3 | 0.089 | 0.390 |
| QRS interval (ms) | 102.4±22.0 | 0.139 | 0.175 |
| QTc (ms) | 423.7±55.3 | 0.251 | 0.013 |
| BMI (kg/m2) | 27.0±3.6 | -0.006 | 0.951 |
| BSA (m2) | 1.8±0.1 | -0.087 | 0.396 |
| LA anteroposterior diameter (mm) | 42.0±4.7 | 0.150 | 0.141 |
| LA mediolateral diameter (mm) | 46.1±6.2 | 0.076 | 0.459 |
| LAVI (ml/m2) | 32.9±12.4 | 0.091 | 0.377 |
| LA apicobasal diameter (mm) | 52.9±7.8 | -0.042 | 0.686 |
| Uric acid (mg/dl) | 6.1±1.36 | 0.085 | 0.412 |
| LVEF (%) | 65.9±7.3 | -0.178 | 0.080 |
| IVS (mm) | 21.6±4.1 | 0.097 | 0.345 |
| PWT (mm) | 12.9±3.1 | -0.034 | 0.738 |
| LVEDD (mm) | 43.0±5.9 | -0.127 | 0.217 |
| LVESD (mm) | 25.5±5.2 | -0.112 | 0.273 |
| LV mass (g) | 330.9±79.0 | -0.008 | 0.938 |
| LVMI (g/m2) | 177.9±48.0 | 0.014 | 0.891 |
| RWTI | 0.6±0.2 | 0.000 | 0.998 |
| Age (year) | 46.8±15.1 | -0.023 | 0.825 |
| LVOTG at rest (mmHg) | 26.6±28.8 | 0.109 | 0.288 |
| LVOTG with Valsalva (mmHg) | 40.6±38.3 | 0.187 | 0.067 |
| MPV (fl) | 9.2±1.5 | -0.003 | 0.975 |
| CRP (mg/l) | 2.8±4.5 | 0.193 | 0.059 |
| ESR (mm/h) | 12.1±14.4 | 0.256 | 0.073 |
BMI: body mass index; BSA: body surface area; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; IVS: interventricular septum; LA: left atrial; LAVI: left atrial volume index; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVMI: left ventricular mass index; LVOTG: left ventricular outflow tract gradient; MPV: mean platelet volume; NLR: neutrophil-to-lymphocyte ratio; QTc: corrected QT interval; PWT: posterior wall thickness; RWTI: relative wall thickness index; SCD: sudden cardiac death; SD: standard deviation.
In bold, statistically significant correlations between the QTc and NLR.
In the present study, we investigated whether NLR could be a predictor of cardiac events. The study's most important findings are the following: (1) NLR was significantly higher in HCM patients compared to age- and gender-matched controls; (2) a high NLR appears to be associated with ventricular arrhythmic events in patients with HCM; (3) NLR was significantly associated with QTc interval; (4) a high NLR appears to be associated with the presence of fQRS; and (5) NLR appears to be significantly higher in the high-risk group, those who demonstrated a predicted SCD risk of over 6%.
In the past few years, several studies have been conducted on NLR in patients with both cardiac and noncardiac diseases.8,9 Activated neutrophils represent nonspecific systemic inflammation, and lymphocytes are a marker of the immune system physiological stress response. NLR integrates these two important and opposite immune pathways, and thus serves as a measure of both systemic inflammation and stress response.10 A high NLR has been associated with poor prognosis in most of these studies. In particular, inflammation plays a key role in the initiation and progression of cardiovascular disease. A previous study showed a correlation between NLR and severity of chronic heart failure. NLR may reflect increased sympathetic drive, and therefore oxidative stress and cytokine production, because of the possible influence of the autonomic nervous system on leukocyte subtype distribution.11 Activated neutrophils are known to play a direct role by adhering to and penetrating the ventricular myocardium and releasing reactive oxygen species, cytokines, and hydrolytic enzymes.12 This leads to extracellular matrix remodeling and reparative processes.13 It was perhaps for this reason that LVEF was under 60% in patients with high NLR in the present study.
The precise mechanism of this inflammatory pathway remains unknown; systemic inflammation can lead to infiltration of the myocardium with inflammatory cells, and this condition may cause irregular interstitial fibrosis in atrial and ventricular tissue. Relationships have been demonstrated between the development of atrial fibrillation and interleukin 8, interleukin 6 (IL-6) and high-sensitivity C-reactive protein (hs-CRP) levels, and increased white blood cell (WBC) count.14 Leukocytes release pro-inflammatory cytokines, such as tumor necrosis factor-alpha, IL-6, and C-reactive protein, and can activate neutrophils to release a variety of proteolytic enzymes such as myeloperoxidase, acid phosphatase, and elastase, which facilitate increased tissue destruction and can lead to direct deleterious effects on the myocardium, resulting in irregular fibrous thickening and the formation of arrhythmia foci in the ventricles.15 NLR has been linked to increased risk of ventricular arrhythmias during percutaneous coronary intervention (PCI).10 Chatterjee et al. reported that pre-procedural elevated WBC count, neutrophilia, and elevated NLR were significant predictors of ventricular arrhythmia in patients undergoing PCI.10 In the present study, NLR was higher in patients with VT, a finding consistent with previous studies. This result suggests that more attention should be paid to the possibility of ventricular arrhythmias in patients with high NLR.
The role of QT and QTc prolongation and fQRS in HCM patients has been studied.16–18 Changes in the myocardium caused by LVH lead to heterogeneity in action potentials and to increased duration of repolarization. These result in an increase in QT or QTc interval.19 Kulan et al. demonstrated a significant relation between QTc interval and LVH.20 Similarly, Gray et al. showed that a QTc interval ≥439 ms, independently of the presence of conventional risk factors, predicts appropriate implantable cardioverter-defibrillator (ICD) therapies in HCM patients, with a more than three-fold increase in the risk of appropriate therapies compared to those without prolonged QT.17 It is possible that a separate mechanism (e.g. myocardial fibrosis) may account for QTc prolongation in HCM. In the present study, we observed longer QTc intervals in patients with high NLR. Likewise, it is known that fQRS complexes in multiple ECG leads are associated with myocardial scarring or fibrosis.5,16 Such changes in the myocardium may lead to heterogeneous ventricular activation, which could manifest as fQRS on ECG.5,20 Fractionated electrograms consisting of multiple discrete deflections have been observed in regions where islands of viable myocardial tissue are interspersed with abundant fibrous tissue. In the present study, NLR was significantly higher in patients with fQRS.
The current European guidelines on HCM recommend a practical risk prediction model for SCD in patients with HCM.5 This model, known as HCM Risk-SCD, is relatively simple and easy to calculate and helps guide therapeutic decision-making. According to this method, a predicted five-year SCD risk of over 6% indicates that the patient is at high risk and an ICD should be considered. In the present study, we observed that NLR was significantly higher in patients with a predicted five-year SCD risk over 6%. As discussed above, nonsustained VT caused by irregular myocardial fibrosis may be responsible for higher SCD risk. Our findings indicate that five-year SCD risk may be higher in patients with high NLR.
Study limitationsThis was not an epidemiological or randomized study. The sample size (n=97) of HCM patients was relatively small. We included only adult HCM patients; elite athletes and individuals with metabolic diseases (e.g. Anderson-Fabry disease) and relevant syndromes (e.g. Noonan syndrome) were excluded. Inflammatory parameters such as IL-6, tumor necrosis factor-alpha, hs-CRP, and myeloperoxidase were not simultaneously measured and compared with NLR because they are generally not available in daily practice. We were unable to examine all of our patients with CMRI because some patients had pacemakers and some refused to participate.
ConclusionsIt is well known that inflammation contributes to progression of cardiac dysfunction and plays a role in worsening of clinical status. Calculation of NLR is a noninvasive, inexpensive, and readily available tool. NLR was significantly higher in patients with HCM compared to the control group. A high NLR is associated with a higher five-year risk of SCD in patients with HCM.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThe authors received no financial support for the research, authorship, and/or publication of this article.
Conflicts of interestThe authors have no conflicts of interest to declare.