Baroreflex function is an independent marker of prognosis in heart failure (HF). However, little is known about its relation to response to cardiac resynchronization therapy (CRT). The aim of this study is to assess arterial baroreflex function in HF patients who are candidates for CRT.
MethodsThe study population consisted of 25 patients with indication for CRT, aged 65±10 years, NYHA functional class ≥III in 52%, QRS width 159±15 ms, left ventricular ejection fraction (LVEF) 29±5%, left ventricular end-systolic volume (LVESV) 150±48 ml, B-type natriuretic peptide (BNP) 357±270 pg/ml, and peak oxygen consumption (peak VO2) 18.4±5.0 ml/kg/min. An orthostatic tilt test was performed to assess the baroreflex effectiveness index (BEI) by the sequence method. This group was compared with 15 age-matched healthy individuals.
ResultsHF patients showed a significantly depressed BEI during tilt (31±12% vs. 49±18%, p=0.001). A lower BEI was associated with higher BNP (p=0.038), lower peak VO2 (p=0.048), and higher LVESV (p=0.031). By applying a cut-off value of 25% for BEI, two clusters of patients were identified: lower risk cluster (BEI >25%) QRS 153 ms, LVESV 129 ml, BNP 146 pg/ml, peak VO2 19.0 ml/kg/min; and higher risk cluster (IEB ≤25%) QRS 167 ms, LVESV 189 ml, BNP 590 pg/ml, peak VO2 16.2 ml/kg/min.
ConclusionsCandidates for CRT show depressed arterial baroreflex function. Lower BEI was observed in high-risk HF patients. Baroreflex function correlated closely with other clinical HF parameters. Therefore, BEI may improve risk stratification in HF patients undergoing CRT.
O barorreflexo arterial é comprovadamente um marcador independente de prognóstico na IC. Contudo, pouco se sabe sobre a relação entre a função do barorreflexo e a resposta à TRC. Assim, o objetivo deste estudo é avaliar a função barorreflexa em doentes com IC candidatos a TRC.
MétodosA população deste estudo prospetivo consistiu em 25 doentes com 65±10 anos, classe NYHA ≥III em 52%, QRS 159±15 ms, fração de ejeção do ventrículo esquerdo (FEVE) 29±5%, volume telessistólico do ventrículo esquerdo (VTSVE) 150±48 mL, péptido natriurético tipo-B (BNP) 357±270 pg/ml, consumo máximo de oxigénio (VO2 max) 18,4±5,0 ml/kg/min. Foi implementado um teste de ortostatismo passivo para avaliar o índice de eficácia do barorreflexo (IEB), através do método sequencial. O grupo controlo foi constituído por 15 indivíduos saudáveis emparelhados para a idade.
ResultadosOs doentes com IC apresentaram um IEB significativamente reduzido durante o tilt (31±12% versus 49±18%, p=0,001). Um IEB diminuído associou-se a um BNP elevado (p=0,038), a um VO2 diminuído (p=0,048) e a um VTSVE aumentado (p=0,031). Aplicando um cut-off 25% para o IEB, foram identificados dois clusters de doentes: cluster de risco menor risco (IEB>25%) QRS 153 ms, VTSVE 129 mL, BNP 146 pg/mL, VO2 max 19,0 mL/kg/min; cluster de maior risco (IEB≤25%) QRS 167 ms, VTSVE 189 mL, BNP 590 pg/mL, VO2 max 16,2 mL/kg/min.
ConclusõesDoentes candidatos a TRC apresentam barorreflexo deprimido. O BEI diminuído foi observado nos doentes de maior risco. O barorreflexo correlacionou-se bem com outros parâmetros de gravidade de IC. Desta forma, o BEI pode contribuir para a estratificação de risco dos doentes com IC submetidos a TRC.
angiotensin-converting enzyme
autonomic nervous system
baroreflex effectiveness index
brain-type natriuretic peptide
blood pressure
cardiac resynchronization therapy
heart failure
heart rate
heart rate variability
left bundle branch block
left ventricular
left ventricular end-diastolic volume
left ventricular ejection fraction
left ventricular end-systolic volume
number of baroreflex events per minute
New York Heart Association
R-R interval on electrocardiogram
systolic blood pressure
oxygen consumption
Chronic heart failure (HF) is a clinical syndrome that affects millions of people worldwide1 and is responsible for high mortality and morbidity and decreased quality of life.2 Although HF affects 1% of the adult population, its prevalence reaches 6-10% of people over the age of 65 years1 and it is responsible for at least 20% of all hospital admissions among the elderly.3 Nearly 30% of patients with HF with decreased ejection fraction also present left ventricular (LV) electrical dyssynchrony that results in a QRS interval greater than 120 ms, most commonly with a left bundle branch block (LBBB) pattern.4 LV electrical dyssynchrony results in decreased diastolic time, anomalous septal motion and increased mitral regurgitation, with an overall reduction in left ventricular ejection fraction (LVEF).1,5
Cardiac resynchronization therapy (CRT) has shown important clinical benefits in the treatment of HF patients with systolic dysfunction (LVEF <35%) and electrical dyssynchrony (QRS >120 ms).4,6 By reducing ventricular electrical dyssynchrony, CRT improves LV systolic function while reducing myocardial oxygen consumption,7 which results in improvement of symptoms and quality of life and in a significant reduction in mortality.4,8,9 However, up to 30% of patients do not respond to CRT (nonresponders).10–12 CRT responders undergo LV reverse remodeling, which is characterized by decreases in intraventricular conduction delay and LV end-systolic volume (LVESV) and reduction in mitral regurgitation area, with a consequent increase in LVEF.8,12,13 LV reverse remodeling is thought to be responsible for the clinical improvement in responders to CRT, but the mechanisms underlying reverse remodeling are not well understood.
The autonomic nervous system (ANS) may play an important role in reverse remodeling. The ANS has an important role in cardiovascular functional and structural regulation,14,15 but ANS function is also recognized as a prognostic marker in HF.16 Furthermore, CRT induces a reduction in mean heart rate (HR) and an increase in heart rate variability (HRV).17–19 Najem et al. also showed that there was an acute increase in cardiac sympathetic activity after biventricular pacing was switched off.20 More recently, an improvement in cardiac sympathetic activity has been demonstrated in responders to CRT, assessed by 123I-MIBG scintigraphy.21,22
Nevertheless, little is known of the impact of CRT on arterial baroreflex function. The arterial baroreflex (with afferents from aortic and carotid baroreceptors) is crucial in the homeostatic regulation of blood pressure (BP).23 It is also a well-established independent prognostic marker in HF; there is evidence that low baroreflex sensitivity is associated with increased cardiovascular morbidity and overall mortality.24 However, it is not known whether baroreflex function before CRT correlates with severity of HF or with the response to cardiac resynchronization. Therefore, the aim of the present study was to assess baroreflex function in HF patients who were candidates for CRT, and to compare it with other clinical and laboratory parameters of HF severity.
MethodsPopulationPatients of both sexes with class I recommendation for CRT were included. We thus included patients in sinus rhythm with symptomatic HF refractory to optimal medical treatment, in New York Heart Association (NYHA) functional class II or III or outpatients in class IV, with LVEF <35% and QRS width >120 ms.6 Patients in NYHA class II with wider QRS and lower LVEF (QRS >130 ms and LVEF <30%) were included.6,13 Patients with atrial fibrillation/flutter, second or third degree atrioventricular block or frequent supraventricular or ventricular ectopic beats, and those with a pacing rhythm were excluded from this study, as sinus rhythm is a prerequisite for reliable arterial baroreflex measurement.25 Patients were recruited at the Cardiology Department of Santa Marta Hospital. All patients provided written informed consent according to the principles of the Helsinki Declaration.
ProtocolThis was an exploratory clinical study. Before CRT implantation, all patients underwent clinical assessment including NYHA functional class, laboratory testing including determination of brain-type natriuretic peptide (BNP), a 12-lead electrocardiogram (ECG) (Philips TRIM III), transthoracic echocardiogram and cardiopulmonary exercise test (CPET). An orthostatic tilt test was used to study baroreflex function.
Transthoracic echocardiographyTransthoracic echocardiographic images were obtained using Vivid 7 equipment (General Electric-Vingmed, Milwaukee, WI). The echocardiographic assessment was performed in M-mode, biplane and Doppler modes. LVEF, LVESV and LV end-diastolic volume (LVEDV) were determined using Simpson's biplane method.26,27 A semi-quantitative Doppler method was used to assess mitral regurgitation grade.
Cardiopulmonary exercise testPatients were instructed to avoid any intense physical activity and not to smoke or ingest any caffeine-containing drinks in the three hours preceding the exam. CPET was performed on a treadmill according to the modified Bruce protocol.28 Patients exercised until maximal fatigue was reached.29 During the test an ECG and a respiratory mass spectrometer with capnography were used to continuously monitor HR, ventilation, oxygen consumption and carbon dioxide production. Using these, peak HR and peak oxygen consumption (peak VO2) were determined.
Orthostatic tilt test and baroreflex function assessmentTilt test protocolOrthostatic tilt testing was performed in a temperature-controlled autonomic function lab. Patients were instructed to avoid any kind of intense physical activity before the test and to avoid smoking or drinking any caffeine-containing drinks in the three hours preceding the exam. No peripheral venous catheters were used and no drugs were administered during the entire exam. All subjects underwent a 10-min basal resting period in the supine position, or slightly inclined in accordance with individual sleeping habits to prevent respiratory discomfort.25 Subjects were tilted up to 70° using a passive electronic tilt table. The orthostatic period was maintained for 10 min, after which subjects returned to their basal inclination for a 10-min recovery period.30,31
Data acquisition and analysisECG and peripheral BP were continuously and noninvasively recorded during the tilt test (Task Force Monitor, CNSystems, Graz, Austria). Hemodynamic data were then analyzed using software developed in our lab (FisioSinal32). This system uses an algorithm to detect systolic blood pressure (SBP) peaks and R-wave peaks in each QRS complex of the ECG from which the RR interval can be calculated. In this way, signals depicting the evolution of SBP over time (systogram) and RR interval (tachogram) were reconstructed.33,34 The reconstructed signals were used to calculate the baroreflex effectiveness index (BEI).
Baroreflex effectiveness index measurementTo assess arterial baroreflex function, the BEI was calculated by the sequence method.35,36 Briefly, the BEI is calculated on the basis of beat-to-beat analysis of cardiovascular signals. The algorithm identifies SBP ramps of three or more consecutive beats characterized by a progressive increase (up-ramp) or decrease (down-ramp) of at least 1 mmHg. Spontaneous baroreflex sequences (baroreflex events) were defined as SBP ramps followed by concomitant and concordant lengthening or shortening of RR intervals of at least 5 ms. The RR intervals were scanned with a lag from the SBP ramp of 0, 1 or 2 beats, each sequence being included only once.36 Baroreflex events were only considered reliable when there was a strong correlation between SBP ramps and concomitant RR-interval ramps (correlation coefficient >0.835). The total number of baroreflex events per minute (NBR) of the analysis was calculated, as well as the BEI, which is defined as the ratio between baroreflex events and the total number of SBP ramps. The higher the BEI, the greater the effectiveness of the arterial baroreflex in hemodynamic adaptation to changes in blood pressure.36,37
As assessment of baroreflex function is ideally performed under stationary conditions,37 baroreflex analysis was divided into three periods for each patient:
- 1)
Basal: 5 min of basal period in supine position;
- 2)
Tilt: 10 min at 70° orthostatic position;
- 3)
Recovery: 5 min of recovery in basal supine position.
The statistical analysis was performed in SPSS software (version 20, IBM, USA). Categorical variables were expressed as percentages or frequencies and continuous variables as means ± standard deviation. The Kolmogorov-Smirnov test was used to test the normal distribution of continuous variables. The non-parametric Mann-Whitney test was used to compare groups. Pearson's correlation coefficient was applied to test the correlation between BEI and the other continuous variables used to describe HF severity. The division of patients according to HF severity was performed by k-means clustering. A p value <0.05 was considered statistically significant.
ResultsPopulationIn this study, 25 HF patients with a mean age of 65±10 years were included, of whom 20% were female. Table 1 summarizes the patients’ demographic and clinical characteristics. In approximately half of the population (13 patients, 52%) ischemic cardiomyopathy was the etiology of HF, the remaining 12 (48%) patients having HF of other causes. In total, 11 patients were in NYHA functional class II, 13 in NYHA class III and one in class IV. Mean QRS duration was 159±15 ms. All patients had depressed systolic function with severely dilated left cardiac chambers (mean values: LVEF 29±5%, LVESV 150±48 ml and LVEDV 207±60 ml). The population was also characterized by a significant limitation of physical activity, as demonstrated by a mean peak VO2 of 18.4±5.0 ml/kg/min. Mean BNP was 357±270 pg/ml. Most patients had multiple comorbidities and other cardiovascular risk factors, notably hypertension (88%), dyslipidemia (80%), diabetes (32%), obesity (28%) and chronic kidney disease (20%). All patients were under optimal medical treatment (Table 2).
Clinical characteristics of heart failure patients who were candidates for cardiac resynchronization therapy.
| n | 25 |
| Female | 5 (20%) |
| Age (years) | 65±10 |
| HF etiology | |
| Ischemic | 13 (52%) |
| Non-ischemic | 12 (48%) |
| NYHA class II/III/IV | 11/13/1 |
| QRS duration (ms) | 159±15 |
| LVEF (%) | 29±5 |
| LVESV (ml) | 150±48 |
| LVEDV (ml) | 207±60 |
| Peak VO2(ml/kg/min) | 18.4±5.0 |
| BNP (pg/ml) | 357±250 |
BNP: brain-type natriuretic peptide; HF: heart failure; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; NYHA: New York Heart Association; VO2: oxygen consumption.
Comorbidities and pharmacologic treatment.
| Comorbidities | |
| Hypertension | 88% |
| Dyslipidemia | 80% |
| Diabetes | 32% |
| Obesity | 28% |
| Chronic kidney disease | 20% |
| Medication | |
| ACE inhibitors/ARBs | 96% |
| Beta-blockers | 96% |
| Diuretics | 80% |
| Spironolactone | 52% |
| Digoxin | 12% |
ACE: angiotensin-converting enzyme; ARBs: angiotensin receptor blockers.
The group of patients were compared with a control group of 15 age-matched individuals (age 58±8 years, p=0.08). In the control group, eight individuals (53%) were female. None had a history of cardiorespiratory disease or dysautonomia or was taking cardiovascular medication.
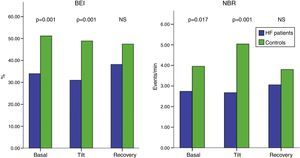
Baroreflex functionBaroreflex function was significantly depressed in HF patients compared to controls. Figure 1 summarizes baroreflex function in HF patients in terms of NBR and BEI. It shows a significant reduction of NBR in HF patients both during the basal period (NBR HF: 2.7±2.3 vs. NBR controls: 3.9±1.6 events/min, p=0.017), and during tilt (NBR HF: 2.7±2.8 vs. NBR controls: 5.0±2.5 events/min, p=0.001). Moreover, HF patients had a lower BEI. In basal supine position, patients’ BEI (34±14%) was significantly lower (p=0.001) than controls’ (51±15%). Similar results were found for the orthostatic tilt period (BEI HF: 31±12% vs. BEI controls: 49±18%, p=0.001). Interestingly, no differences in baroreflex function were found during recovery.
Baroreflex function in candidates for cardiac resynchronization therapy (green) compared to healthy controls (blue), measured by BEI and NBR. Heart failure patients have significant decreased baroreflex function. Statistical significance p<0.05 (Mann-Whitney test). BEI: baroreflex effectiveness index; NBR: number of baroreflex events per minute; NS: non-significant.
Of all the parameters assessed, the following were selected to better characterize HF severity: NYHA functional class, QRS duration, BNP, peak VO2, LVEF, LVESV, LVEDV and mitral regurgitation grade. Table 3 shows the calculated correlation coefficients between the above variables and NBR or BEI. During tilt, a lower BEI was associated with higher BNP values (r=-0.435, p=0.038), higher LVESV (r=-0.541, p=0.031) and lower peak VO2 (r=0.472, p=0.048) (Figure 2). Furthermore, there was a significant negative correlation between basal BEI and BNP (r=-0.505, p=0.033), as well as between NBR during tilt and BNP (r=-0.517, p=0.018), demonstrating that depressed baroreflex function is associated with increased BNP values. During tilt, the associations between BEI and LVEDV (r=-0.486, p=0.056) and between NBR and peak VO2 were non-significant (r=0.428, p=0.077).
Correlation between baroreflex function and parameters of heart failure severity.
| Baroreflex function | NYHA class | QRS | BNP | Peak VO2 | LVEF | LVESV | LVEDV | MR | |
|---|---|---|---|---|---|---|---|---|---|
| Basal BEI | Pearson's r | -0.177 | -0.19 | -0.505 | 0.206 | 0.022 | -0.221 | -0.129 | -0.111 |
| p | 0.431 | 0.398 | 0.033 | 0.428 | 0.926 | 0.428 | 0.646 | 0.65 | |
| Tilt BEI | Pearson's r | -0.251 | -0.287 | -0.435 | 0.472 | 0.195 | -0.541 | -0.486 | -0.021 |
| p | 0.248 | 0.184 | 0.038 | 0.048 | 0.385 | 0.031 | 0.056 | 0.931 | |
| Basal NBR | Pearson's r | -0.059 | -0.071 | -0.314 | 0.256 | 0.193 | -0.314 | -0.243 | -0.162 |
| p | 0.779 | 0.736 | 0.191 | 0.304 | 0.366 | 0.22 | 0.348 | 0.482 | |
| Tilt NBR | Pearson's r | -0.265 | -0.187 | -0.537 | 0.428 | 0.354 | -0.392 | -0.331 | 0.007 |
| p | 0.201 | 0.371 | 0.018 | 0.077 | 0.09 | 0.12 | 0.195 | 0.977 | |
BEI: baroreflex effectiveness index; BNP: brain-type natriuretic peptide; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; MR: mitral regurgitation; NBR: number of baroreflex events per minute; NYHA: New York Heart Association.
A lower BEI was associated with higher BNP values, lower peak oxygen consumption and increased cardiac volumes. Statistical significance p<0.05 (Pearson's r linear correlation coefficient). BEI: baroreflex effectiveness index; BNP: brain-type natriuretic peptide; LVESV: left ventricular end-systolic volume; VO2: oxygen consumption.
In view of the above results, BEI during tilt was used to stratify patients, as it was the baroreflex function measurement that correlated with most HF severity variables (LVESV, BNP and peak VO2). Applying k-means clustering, a cut-off value of 25% for BEI during tilt was found to stratify patients into two clusters of different HF severity (Figure 3): a low-risk cluster (BEI >25%) with QRS duration of 153 ms, LVESV 129 ml, BNP 146 pg/ml, and peak VO2 19.0 ml/kg/min; and a high-risk cluster (BEI ≤25%) with QRS duration 167 ms, LVESV 189 ml, BNP 590 pg/ml, and peak VO2 16.2 ml/kg/min. These clusters demonstrate that baroreflex function can be used to better stratify HF patients who are candidates for CRT.
Applying a cut-off value of 25% for BEI, two HF risk clusters were obtained. In cluster 2 HF patients who present low baroreflex function have wider QRS complexes, higher LVESV and BNP values and reduced functional status as shown by peak VO2. This is therefore the patient group with higher HF clinical risk (K-means clustering, variables expressed as means). BEI: baroreflex effectiveness index; BNP: brain-type natriuretic peptide; HF: heart failure; LVESV: left ventricular end-systolic volume; VO2: oxygen consumption.
Our results show that HF patients who are candidates for CRT have depressed baroreflex function. This is in agreement with the literature, since this population consisted of selected HF patients and HF is a syndrome classically characterized by compensatory chronic neurohormonal hyperstimulation, with hypersympathetic activation and attenuation of the arterial baroreflex.38,39 The extent of the reduction in baroreflex function has important prognostic value in HF, lower values of baroreflex sensitivity being associated with increases in overall mortality, cardiovascular morbidity and mortality and HF hospital admissions.24 Moreover, BEI correlated well with other variables of HF severity. Patients with lower BEI were those with increased cardiac volumes (LVESV and LVEDV), increased BNP and lower functional capacity (peak VO2). BNP and peak VO2 are two independent prognostic markers in HF.28,40 They are widely used in risk stratification of HF patients,40 with important implications for HF clinical management and treatment, including the utility of peak VO2 in stratifying patients for cardiac transplantation.41 Besides the importance of baroreflex function as a prognostic marker in HF,24 our results show that it correlates well with other risk stratification variables including BNP and peak VO2, supporting the idea that baroreflex function itself may be used in HF risk stratification. We found that BEI during tilt could be used to divide patients into low-risk and high-risk HF groups (Figure 3). In our study, patients with BEI lower than 25% had more severe HF. We suggest further studies to use this cut-off value for BEI in order to identify patients selected for CRT that have more significant HF risk.
The importance of the baroreflex in CRT is still not clear as there are few studies on this subject in the literature. However, Braga et al. reported one patient who fully recovered baroreflex function to normal values three months after CRT.39 Moreover, Gademan et al. demonstrated that there was an acute increase in baroreflex sensitivity and HRV after the implantation of a biventricular pacemaker25 and that this increase was predictive of the response to CRT.42 However, in these studies baroreflex function was assessed only after the implantation of a CRT device,25,42 with biventricular pacing switched on and off. It would be valuable to assess baroreflex function before and after CRT device implantation to better understand the autonomic impact of CRT on cardiovascular function. Moreover, the importance of a predictive index is greater when assessed before implantation. Likewise, there are no studies demonstrating that the increase in baroreflex function is sustained chronically, nor if this increase correlates with cardiac reverse remodeling. Further follow-up studies are necessary to better answer all these questions.
Our methodological approach is based on the sequence method,35 which measures baroreflex function through a non-invasive beat-to-beat measurement of HR and SBP. Although the studies that established the prognostic value of the baroreflex used the classic method for measurement of baroreflex sensitivity (the phenylephrine technique),24 there is significant evidence that non-invasive methods give similar results to those obtained through the phenylephrine technique and obviate the use of vasoactive drugs.43,44 The sequence method is used to calculate BEI, a new baroreflex function index that measures the effectiveness with which the HR responds to SBP variations.36,37,45 BEI is less liable to be influenced by ectopic beats, which were quite frequent in our patients, than baroreflex sensitivity. Recently, the assessment of baroreflex function through non-invasive methods by the use of provocative maneuvers has been extended to different clinical entities including reflex syncope,30,37 atrial fibrillation,31 and HF,25 in which it appears to improve specificity. Our work is the first to use a tilt test in HF patients and the results during the orthostatic period provided more consistent data than during the supine basal period, as BEI during tilt correlated with more HF severity variables. Our approach is innovative in the use of tilt testing in patients with HF and our results show that it is a reproducible, consistent and well-tolerated test.
The size of our study group (n=25) is the main limitation of this work. Moreover, the control group also presents some limitations, as it was age-matched but not gender-matched. Furthermore, the fact that all patients were under optimal medical treatment may also be considered a limitation to a work that aims to assess basal baroreflex function, as 96% of patients were taking a beta-blocking agent. Nevertheless, La Rovere et al. have recently shown in a prospective study that there are no significant differences in baroreflex sensitivity between HF patients treated with beta-blockers and those that are not.46
Our work shows the importance of baroreflex assessment in risk stratification of HF patients who are candidates for CRT. In the future, prospective studies with at least a six-month post-CRT follow-up are needed in order to demonstrate the existence of autonomic remodeling associated with CRT and to assess whether baroreflex measurements have predictive value concerning response to CRT.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.








 BEI and
BEI and  BEI was associated with higher
BEI was associated with higher  BEI, two HF risk clusters were obtained. In cluster 2 HF patients who present low baroreflex function have wider QRS complexes, higher
BEI, two HF risk clusters were obtained. In cluster 2 HF patients who present low baroreflex function have wider QRS complexes, higher 

