The authors present the case of a 68-year-old man with predominantly right heart failure in the context of severe aortic stenosis associated with pulmonary hypertension. Anemia was diagnosed which, after endoscopic study, was considered to be secondary to angiodysplasia and a diagnosis of Heyde syndrome was made. After valve replacement surgery the patient's heart failure improved and hemoglobin levels stabilized.
We present this case to show the need to recognize less common associations of severe aortic stenosis, in order to provide immediate and appropriate treatment.
Os autores apresentam um caso clínico de um homem de 68 anos com clínica de insuficiência cardíaca predominantemente direita, no contexto de estenose aórtica grave associada a hipertensão pulmonar. Concomitantemente, foi diagnosticada anemia que, após estudo endoscópico, se concluiu ser secundária a angiodisplasias intestinais, tendo sido feito o diagnóstico de síndrome de Heyde.
Após cirurgia de substituição valvular houve resolução do quadro, com melhoria dos sintomas de insuficiência cardíaca prévia e estabilização dos valores de hemoglobina.
Com este caso pretende-se mostrar a necessidade do conhecimento de associações menos frequentes na estenose aórtica grave para uma atuação terapêutica imediata e adequada.
A 68-year-old man with type 2 diabetes (medicated with metformin and vildagliptin) and dyslipidemia (medicated with statins and fenofibrate) as cardiovascular risk factors, and a history of alcohol abuse (100 g/day), had been diagnosed with iron deficiency anemia a year previously that was investigated by endoscopy, which revealed chronic atrophic gastritis.
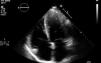
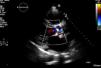
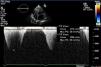
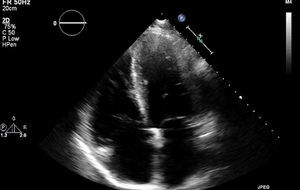
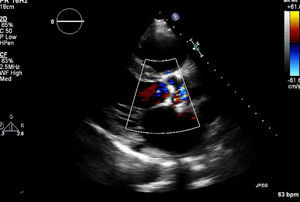
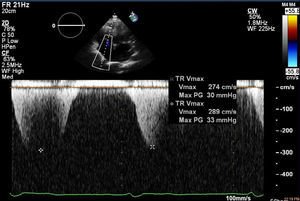
In March 2013 he began to experience fatigue, dyspnea on moderate and mild exertion, abdominal swelling, lower limb edema and cachexia. He was medicated with furosemide and ivabradine and assessed by a cardiologist four months after symptom onset. On physical examination he was in New York Heart Association (NYHA) class III, with marked weight loss and venous jugular distention; bilateral basal rales on pulmonary auscultation; rhythmic S1 and S2 on cardiac auscultation with a grade III/VI aortic early to mid systolic murmur radiating to the carotids; palpable hepatomegaly 4 cm below the costal margin; moderate ascites and lower limb edema up to the knee. The electrocardiogram showed sinus rhythm and complete right bundle branch block. On the chest X-ray cardiomegaly was visible with right atrial dilatation and bilateral hilar enlargement. Laboratory tests revealed anemia (hemoglobin [Hb] 9.9 g/dl). The echocardiogram showed dilatation of both atria and the right ventricle (RV) (Figure 1), with mild hypertrophy of the ventricular septum; a thickened and calcified aortic valve with significantly limited opening (Figure 2); peak left ventricular (LV)/aortic gradient of 55 mmHg, mean 33 mmHg, and functional aortic valve area of 0.8 cm2 (0.39 cm2/m2); mild to moderate tricuspid regurgitation (Figure 3) with pulmonary artery systolic pressure (PASP) of 51 mmHg; mild to moderate LV systolic dysfunction; systolic and diastolic straightening of the ventricular septum; and impaired RV systolic function.
To investigate the signs of pulmonary hypertension (PH) in a patient with severe isolated aortic stenosis (AS), spirometry was performed, which was normal; lung computed tomography showed no sign of pulmonary embolism (PE) or other alterations of the pulmonary parenchyma, while ventilation/perfusion scintigraphy showed a low probability of PE. Abdominal ultrasound revealed moderate ascites, hepatomegaly (19.2 cm), moderate homogeneous splenomegaly (14.7 cm), and no signs of cirrhosis or portal hypertension. The dosage of diuretics was increased and the patient was referred for surgical aortic valve replacement.
His heart failure symptoms (HF) persisted and he was hospitalized three weeks after the initial assessment. Intravenous diuretics led to progressive improvement. Laboratory tests showed Hb 8.6 g/dl, and iron kinetics, vitamin B12 and folic acid levels, activated partial thromboplastin time, prothrombin time and fibrinogen were all normal. Platelet function testing showed increased platelet aggregation time, while levels of von Willebrand factor (vWF) and vWF ristocetin cofactor (RCo) activity (vWF:RCo) were normal. Cardiac catheterization showed RV end-diastolic pressure of 27 mmHg, pulmonary capillary wedge pressure (PCWP) of 27 mmHg, and PASP of 65 mmHg; intracavitary shunts were excluded by oximetry. Coronary angiography revealed non-significant coronary artery disease.
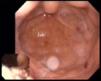
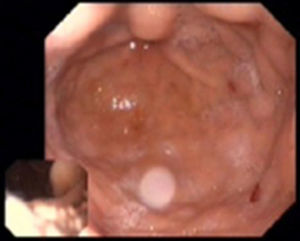
The patient's anemia worsened progressively during hospital stay, with a minimum Hb of 5.3 g/dl, requiring transfusion of eight units of red cell concentrate and intravenous iron supplementation. At this time an endoscopic study revealed two gastrointestinal angiodysplasias (Figure 4) which were treated by argon plasma coagulation. Since the patient's Hb values remained unstable, capsule endoscopy was performed, which revealed active jejunal bleeding, treated with epinephrine hemostasis and argon plasma ablation of jejunal angioectasias by enteroscopy.
Hb levels then stabilized at 9 g/dl and on the 28th day of hospitalization the patient underwent surgical aortic valve replacement with a 23-mm Carpentier-Edwards PERIMOUNT biological valve and De Vega tricuspid annuloplasty, which were uneventful. He was medicated with furosemide, carvedilol, clopidogrel, oral antidiabetic agents and lipid-lowering drugs.
At an outpatient consultation one month after surgery, he was in NYHA class II with no visible evidence of blood loss; Hb levels remained stable at 9.2 g/dl. The echocardiogram revealed moderate left atrial dilatation and normal dimensions of the other chambers, normal appearance of the cusps of the biological aortic valve with no pathological regurgitation (functional area 1.5 cm2), correctly implanted tricuspid valve annulus with no stenosis or regurgitation, and preserved biventricular function.
DiscussionThe association between severe AS and gastrointestinal bleeding was first described in 1958 by Edward Heyde, and is now known as Heyde syndrome (HS).1
Investigation revealed that the bleeding in HS is associated with vascular alterations, particularly angiodysplasias.1
AS in the elderly is usually degenerative and is associated with the same cardiovascular risk factors as atherosclerotic disease.2 It is the most common valve disease in Europe, affecting 2–7% of those aged over 65.2,3
Angiodysplasias are more common in individuals aged over 60 and are responsible for 1–6% of hospitalizations for gastrointestinal bleeding and 30–40% of bleeding of obscure cause.1,4
In HS, AS makes angiodysplasias clinically manifest.4 Gastrointestinal bleeding is 100 times more common in patients with calcified AS than in the general population.5
The rarity of this association and the frequently subjective and varying diagnostic criteria make it difficult to study the epidemiology of the syndrome, the true prevalence of which is unknown.1,6
Various theories have been put forward to explain HS, including a pathophysiology common to AS and angiodysplasia and common cardiovascular risk factors, a predisposition for the formation of angiodysplasias together with the low cardiac output and pulse wave abnormalities arising from AS, and intestinal hypoxia caused by cholesterol emboli or atherosclerotic plaques.1,4,6
The most widely accepted etiology is acquired vWF deficiency due to the mechanical disruption of vWF multimers by the turbulent flow in the stenotic aortic valve, which makes them vulnerable to degradation by metalloproteinases. In addition, this turbulent flow increases the interaction between vWF and platelets and the formation of microaggregates, leading to platelet sequestration and multimer degradation, and is thus frequently associated with thrombocytopenia.1,4,6,7
The severity of vWF deficiency is directly related to the severity of AS as measured by the transvalvular gradient.4,7,8
Through the same pathophysiological mechanism, vWF deficiency may be found in some cases of hypertrophic cardiomyopathy or congenital heart disease.4
The presence of vWF multimers is essential for hemostasis in areas of high blood flow, such as angiodysplasias.1,4 Patients with severe AS thus have higher bleeding risk, especially if taking antiplatelets or undergoing surgical procedures; the increase in risk is related to acquired vWF deficiency, with ratio of collagen-binding activity to antigen reduced in 67% and platelet-function analyzer closure time prolonged in 92% in patients with severe AS.7
A diagnosis of HS is made on the basis of severe AS on echocardiography and gastrointestinal bleeding due to angiodysplasia documented by endoscopy.1,4,6
In decreasing order of sensitivity, the findings that demonstrate acquired vWF deficiency are absence of vWF multimers by electrophoresis; increased platelet aggregation time on platelet function testing; skin bleeding time; vWF:RCo activity and vWF antigen level (values of the latter two are often normal in patients with acquired vWF deficiency secondary to severe AS).4
Treatment of gastrointestinal bleeding includes endoscopic hemostasis, angiographic embolization, intestinal resection,1,6 estrogen-progesterone therapy1,9 (although there have been few case reports or studies on this approach, hormone therapy may be effective in controlling recurrent bleeding from gastrointestinal vascular malformations),9 octreotide,6,10 and intravenous iron supplements and blood transfusions.1,6 Complete resolution of HS is achieved by aortic valve replacement, the angiodysplasias remaining but without bleeding; this is a lasting cure.1,6
Although isolated bleeding in severe AS is not a formal indication for valve replacement, it should be considered on a case-by-case basis.
Biological valves are to be preferred in order to avoid long-term anticoagulation, but this decision will depend on operative risk and the patient's life expectancy.1 Valve replacement is preferable to intestinal resection, both because of the surgical risk due to underlying valve disease and because the intestinal lesions are likely to be dispersed and thus not all can be resected.1,6
In the case presented, the diagnosis of HS was made on the basis of the association of active gastrointestinal bleeding and severe AS. The finding of increased platelet aggregation time in the absence of other causes, even with normal vWF:RCo activity and vWF antigen levels, suggests an acquired vWF deficiency.
There have been few cases described in the literature of the association of severe AS, vWF deficiency and gastrointestinal bleeding.5
The therapeutic approach adopted in this case was similar to that in other case reports.8,10 Maintenance of stable Hb levels, through treatment for the lesions responsible for the bleeding, iron supplementation and blood transfusions, kept the patient clinically stable until a permanent cure could be achieved, reducing surgical risk during valve replacement.
The other unusual feature of this case was the presence of severe PH associated with right chamber dilatation, causing right HF, which is atypical in cases of isolated severe AS.
The presence of a marked increase in PASP associated with elevated PCWP resulting from increased LV end-diastolic pressure, the exclusion of other likely causes, and normalization of right chamber dimensions and resolution of PH after valve replacement, indicate that severe AS was the underlying cause.
The prevalence of PH in AS is 28–56%, and 11–21% of severe PH 11–21%.11 As demonstrated by Cam et al.11 and Malouf et al.,12 valve replacement should be considered in cases of severe PH secondary to severe AS, especially when associated with increased PCWP, as the long-term benefit outweighs the surgical risk.
The specific characteristics of this case required a multidisciplinary approach to stabilizing the patient, and the outcome demonstrated that the benefit of valve replacement outweighed the high surgical risk and that surgery was the only way to achieve lasting clinical improvement.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Godinho AR, Amorim S, Campelo M, et al. Estenose aórtica grave: associações esquecidas. Rev Port Cardiol. 2014;33:563.e1–563.e4.