The aim of this study was to investigate the serum exosome proteome profile of coronary artery dilatation (CAD) caused by Kawasaki disease (KD).
MethodsTwo-dimensional electrophoresis was implemented on proteins of serum exosomes obtained from children with CAD caused by KD and from healthy controls. Differentially expressed proteins were identified by matrix-assisted laser desorption/ionization time-of-flight/time-of-flight mass spectrometry analysis.
ResultsWe identified 38 differentially expressed proteins (13 up-regulated and 25 down-regulated) from serum exosomes of patients with CAD caused by KD compared with healthy controls. Expression levels of three differentially expressed proteins (leucine-rich alpha-2-glycoprotein, sex hormone-binding globulin, and serotransferrin) were validated using western blot analysis. Classification and protein-protein network analysis showed that they are associated with multiple functional groups involved in the acute inflammatory response, defense response, complement activation, humoral immune response, and response to wounding. The majority of the proteins are involved in the inflammation and coagulation cascades.
ConclusionsThese findings establish a comprehensive proteome profile of CAD caused by KD and increase our knowledge of scientific insight into its mechanisms.
O objetivo deste estudo consistiu em investigar o perfil proteico do exossoma sérico da dilatação da artéria coronária (DAC) causada pela doença de Kawasaki (DK).
MétodosImplementámos a tecnologia 2-DE nas proteínas do exossoma proveniente do soro de crianças com DAC causada pela DK e de controlos saudáveis. Foram identificadas proteínas com diferente expressão, analisadas através de espectrometria de massa com técnica de ionização e dessorção a laser assistida por matriz com análise de tempo de voo de aceleração.
ResultadosIdentificámos 38 proteínas diferenciais (13 reguladas positivamente e 25 negativamente) do exossoma sérico da DAC causada pela DK comparadas com os controlos saudáveis. Os níveis de expressão de três proteínas diferenciais (glicoproteína alfa 2 rica em leucina, globulina ligada às hormonas sexuais, serotransferrina) foram validados com a utilização de western blot. A análise da classificação e a rede proteína-proteína mostraram que estão associadas a múltiplos grupos funcionais, incluindo a resposta inflamatória aguda, a resposta de defesa, a ativação do complemento, a resposta imune humoral e a resposta a lesões. A maioria das proteínas estava envolvida no processo da inflamação e cascata de coagulação.
ConclusõesEstes achados estabeleceram um perfil do proteoma abrangente da DAC causado pela DK e aumentam os nossos conhecimentos de base sobre a visão científica dos mecanismos da DAC causada pela DK.
Kawasaki disease (KD) is an acute systemic vasculitis of children that presents with prolonged fever and mucocutaneous inflammation, including inflammation of the oral mucosa, non-exudative conjunctivitis, rash, extremity changes and cervical lymphadenopathy that is usually unilateral.1,2 Delays in accurate diagnosis lead to increased mortality and morbidity from complications. In particular, without timely treatment, as many as 25% of patients may develop coronary artery dilatation or aneurysms, with associated risk of long-term morbidity or death. Importantly, no pathognomonic test exists for the early identification and diagnosis of KD. Therefore, it is crucial to understand the molecular pathogenesis of CAD in KD so as to improve diagnosis and therapy.
Exosomes are secreted by multiple cell types and are found in virtually all body fluids.3 They are nano-sized, cell membrane-surrounded structures harboring a broad range of biomolecules, containing mRNAs, miRNAs and proteins linked to cell type-associated functions.4 Previous reports have demonstrated that exosomes play an important role in cell-to-cell communication and influence both physiological and pathological processes.5,6 The protein composition of exosomes can be investigated using mass spectrometry. This approach allows for an unbiased assessment of the proteome without the need for prior knowledge of protein identities.
In the present study, we employed two-dimensional electrophoresis (2-DE) and matrix-assisted laser desorption ionization (MALDI) time-of-flight mass spectrometry (TOF MS) to compare proteomes in serum exosomes from healthy children and KD patients with CAD, seeking to identify these pathophysiological alterations on a proteomic scale. To our knowledge, this is the first serum exosome proteomic analysis in the context of pathogenesis of CAD caused by KD, providing a comprehensive atlas of the serum exosome proteome of CAD in KD.
MethodsPreparation of serum samplesEthical approval was obtained for human sample collection from the Ethics Committee at Guangzhou Women and Children's Medical Center (trial no. 077 2013), and written informed consent was obtained from all guardians. Blood samples from six KD patients with CAD were randomly selected according to the American Heart Association and the Japanese Ministry of Health and Welfare criteria. The patients underwent systematic examination in the hospital and were confirmed to have no other diseases. Blood samples from six healthy children were used as the control group. Blood samples were separated by centrifugation at 1000 g for 10 min. Serum aliquots were collected and stored at −80°C.
ExoQuick precipitation of serum exosomeExosomes were isolated from two pooled serum samples consisting of equal amounts of each of six experimental samples from KD patients and healthy children using ExoQuick precipitation (System Biosciences Inc, Mountain View, CA) following the manufacturer's instructions.7,8
Exosome characterizationTransmission electron microscopyThe exosome extraction reagents were used to precipitate exosomes from serum samples of KD patients and healthy children, which were then centrifuged at 1500 g for 10 min at 4°C to remove the supernatant. The exosome pellet was resuspended in 10 mM phosphate buffered saline in four times the volume of serum. A copper mesh was placed on a clean wax plate and 100 μl of the exosome suspension was added. After 4 min, the copper mesh was removed and placed in 2% phosphotungstic acid for 5 min. The mesh was laid on the filter paper for drying, and transmission electron microscopy (TEM) was used to observe the morphological features of the exosomes.
Western blot analysisThe exosome pellet was completely dissolved in the protein lysis buffer, and the protein concentration was determined using a Bradford protein assay kit (Bio-Rad, Hercules, CA). The exosome proteins were separated on a 1D SDS-PAGE gel before being transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was incubated with CD9 (1:1000), CD81 (1:1000) and flotillin (1:1000) primary antibodies at 4°C overnight, followed by incubation with the corresponding secondary antibodies at room temperature for 1 h. Specific protein bands were detected using the SuperSignal chemiluminescence detection system (Pierce, Rockford, IL).
Two-dimensional electrophoresisThe prepared pooled protein samples (200 μg protein) were mixed with rehydration buffer to a volume of 450 μl. Immobilized pH gradient (IPG) strips (pH 4–7, 24 cm, GE Healthcare) for the first dimension were used to isolate the altered proteins and the following parameters were set at 20°C: 300 V for 30 min, 700 V for 30 min, 1500 V for 1.5 h, 9900 V for 3 h, 9900 V for 6.5 h, 600 V for 20 h, and 8000 V constant voltage for a total of 56 kVh. After isoelectric focusing, the strips were equilibrated in two steps: 15 min in an IPG equilibration buffer (6 M urea, 2% SDS, 30% glycerol, 0.375 M Tris, 20 mg/ml DTT, and a trace of bromophenol blue), and then alkylated for 15 min. Subsequently, a 12.5% SDS-PAGE gel was applied. Electrophoresis was carried out at 20 mA per gel for 40 min and then at 30 mA per gel until the dye front reached the bottom. The protein spots were detected by either Coomassie Brilliant Blue G-250 staining or silver staining.
Image analysisThe gels were analyzed in ImageMaster 2D Platinum software (GE Healthcare). The normalized protein amount for each spot was calculated as the ratio of that spot volume to the total spot volume on the gel. A threshold of 2.0-fold (p<0.05) change was used to determine significant differences between groups.
In-gel digestionEach gel piece was washed three times with deionized water, destained with 50% methanol for 30 min at 37°C, and dehydrated in 100 μl of acetonitrile (ACN) for 20 min at room temperature. The gel spots were then swollen in 50 μl of 100 mM NH4HCO3, dehydrated for a second time, and incubated in 1 μg/50 μl trypsin (Promega) for 30 min at 4°C. Next, cover solution (10% acetonitrile, 50 mM NH4HCO3, deionized water) was added and the samples were incubated for 16 h at 37°C. The peptide mixtures were extracted using 2.5% trifluoroacetic (TFA)/90% ACN for 30 min at room temperature and completely dried in a Speed Vac centrifuge.
Protein identificationAfter digestion, the tryptic peptides were dissolved in 1.5 μl solution containing deionized water, 50% ceric ammonium nitrate, and 0.1% trifluoroacetic acid. Then, 0.8 μl mixture was spotted onto a MALDI target plate using 0.5 μl HCCA (5 mg/ml a-Cyano-4-hydroxycinnamic acid) matrix. Peptide mass spectra were analyzed with a MALDI TOF mass spectrometer (Applied Biosystems, USA). The UV laser was operated at a 200 Hz repetition rate with an acceleration voltage of 20 kV and a wavelength of 355 nm.
Database searchThe mass spectrometer data were matched to a corresponding virtual peptide mass database derived from GPS Explorer™ version 3.6, the UniProt database, and the Mascot search engine with the Homo sapiens taxonomy. Protein identification was performed by peptide mass fingerprint (PMF) using Mascot software (http://www.matrixscience.com). The search parameters used in PMF were as follows: species: Homo sapiens; fixed modifications: carbamidomethylation; enzyme: trypsin; variable modifications: oxidation (M). The function, gene name, and Gene Ontology category of each protein were determined with the UniProt Homo sapiens protein database and the Mascot v2.1 software protein database search engine.
Western blot analysisProtein extracts from the exosomes of six KD patients and six healthy children serum samples were separated using SDS-PAGE, and then transferred onto PVDF membranes. The membranes were incubated with sex hormone-binding globulin (SHBG) (1:1000), leucine-rich alpha-2-glycoprotein (LRG1) (1:1000) and serotransferrin (TF) (1:1000) antibodies at 4°C overnight, followed by incubation with secondary antibodies at room temperature for 2 h. The bands were detected using the SuperSignal chemiluminescence detection system (Pierce, Rockford, IL).
Protein categorizationIdentified proteins were classified based on the Database for Annotation, Visualization and Integrated Discovery (DAVID) (http://david.abcc.ncifcrf.gov/). DAVID consists of an integrated biological knowledge base and a comprehensive set of analytic tools to extract biological themes from large gene/protein lists, such as those derived from genomic and proteomic studies. The significance cutoff parameter was estimated by Fisher's exact test.
Prediction of protein-protein interactionsThe differentially expressed proteins were processed by the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) system (http://www.string-db.org). STRING is a database of known and predicted protein interactions, including direct (physical) and indirect (functional) associations.
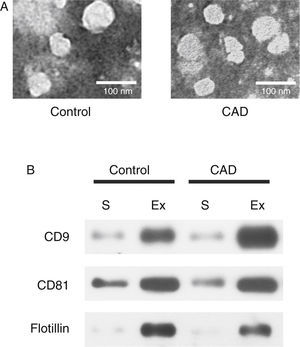
ResultsExosome validationMicrovesicles were separated from the serum of healthy controls and KD patients with CAD with ExoQuick precipitation reagent. TEM and western blot were used in validation of exosomes. Under TEM, the morphological structures of the isolated microvesicles were 30–100 nm in diameter (Figure 1A). The expression levels of the three specific exosome biomarkers (CD9, CD81, and flotillin) were markedly higher in isolated microvesicles than in serum (Figure 1B). Both results confirmed that the microvesicles separated from serum were indeed exosomes.
Characterization of exosomes in serum samples from Kawasaki disease patients with coronary artery dilatation (CAD) and healthy children by transmission electron microscopy and western blot. (A) Morphological characterization by transmission electron microscopy; (B) molecular characterization by western blot. Protein extracts prepared from sera (S) or exosomes (Ex) were examined using antibodies against exosomal protein markers (CD9, CD81 and flotillin).
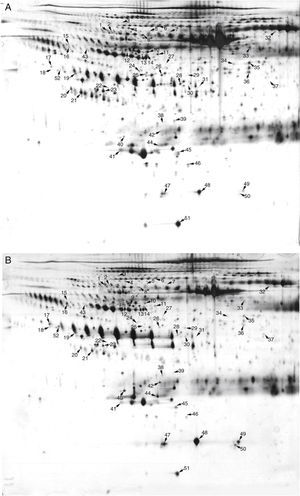
Pooled proteins from serum exosomes of six KD patients with CAD and six healthy children were separated by 2-DE (Figure 2A and 2B). After visual review, 52 differential protein spots with at least a 2.0-fold discrepancy between the two groups were selected for MALDI-TOF/TOF MS analysis. In total, 38 differentially expressed proteins (13 up-regulated and 25 down-regulated) were successfully identified (Table 1). The unidentified proteins likely had special physical and chemical properties, or ambiguous identifications, or low Mascot scores. Some proteins appeared as multiple spots in 2-DE, likely due to modifications and/or the presence of isoforms.
Two-dimensional electrophoresis analysis of the serum exosome proteome. Gels were visualized by silver staining. Arrows indicate differentially expressed proteins in Kawasaki disease (KD) patients with coronary artery dilatation (CAD) and healthy controls. Spot numbers correspond to proteins in Table 1. (A) Healthy controls; (B) KD patients with CAD.
Identification of differentially expressed proteins from patients with coronary artery dilatation caused by Kawasaki disease by matrix-assisted laser desorption/ionization time-of-flight/time-of-flight mass spectrometry analysis.
| Spot no. | Protein name | Accession no. | Protein MW (kDa) | Protein pI | CAD/C | Protein score |
|---|---|---|---|---|---|---|
| 1 | 35 kDa inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4) | Q14624 | 103521 | 6.51 | 2.54267 | 224 |
| 2 | Vitamin K-dependent protein S (PROS1) | P07225 | 74316 | 5.42 | −2.20672 | 298 |
| 3 | Complement component C9 (C9) | P02748 | 61728 | 5.42 | 2.04505 | 194 |
| 4 | Afamin (AFM) | P43652 | 70963 | 5.64 | −2.58006 | 348 |
| 5 | Insulin-like growth factor-binding protein complex acid labile subunit (IGFALS) | P35858 | 66735 | 6.33 | −3.25489 | 197 |
| 6 | Complement C4-A (C4A) | P0C0L4 | 84758 | 5.33 | −2.16898 | 229 |
| 7 | Hemopexin (HPX) | P02790 | 52385 | 6.55 | −1000000 | 82 |
| 8 | Alpha-1B-glycoprotein (A1BG) | P04217 | 52479 | 5.65 | −2.20039 | 314 |
| 9 | Inter-alpha (globulin) inhibitor H4 (plasma kallikrein-sensitive glycoprotein) variant | Q59FS1 | 76971 | 5.72 | 2.26848 | 390 |
| 10 | Antithrombin-III (SERPINC1) | P01008 | 49350 | 5.95 | −3.45503 | 476 |
| 11 | Antithrombin-III (SERPINC1) | P01008 | 49350 | 5.95 | −2.14968 | 330 |
| 12 | Angiotensinogen (AGT) | P01019 | 53406 | 5.87 | 2.78334 | 246 |
| 13 | Inter-alpha (globulin) inhibitor H4 (plasma kallikrein-sensitive glycoprotein) variant | Q59FS1 | 76971 | 5.72 | 3.02175 | 330 |
| 14 | Vitamin D-binding protein (GC) | P02774 | 52807 | 5.22 | −2.81622 | 241 |
| 15 | Kininogen-1 (KNG1) | P01042 | 48936 | 6.29 | −1000000 | 274 |
| 16 | Alpha-1-antichymotrypsin (SERPINA3) | P01011 | 45567 | 5.32 | 2.86774 | 270 |
| 17 | Leucine-rich alpha-2-glycoprotein (LRG1) | P02750 | 38382 | 6.45 | 3.23996 | 358 |
| 18 | Complement C4-B (C4B) | P0C0L5 | 40795 | 5.19 | 4.87092 | 137 |
| 19 | Haptoglobin (HP) | P00738 | 38868 | 6.26 | 4.89816 | 323 |
| 20 | Clusterin (CLU) | P10909 | 49342 | 6.27 | −4.18545 | 230 |
| 21 | Clusterin (CLU) | P10909 | 49342 | 6.27 | −2.82043 | 329 |
| 22 | Serum paraoxonase/lactonase 3 (PON3) | Q15166 | 39810 | 5.24 | −2.55977 | 205 |
| 23 | Complement C3 (C3) | P01024 | 104599 | 5.16 | −2.22021 | 375 |
| 24 | Vitamin D-binding protein (GC) | P02774 | 52807 | 5.22 | −2.09703 | 154 |
| 25 | CD5 antigen-like (CD5L) | O43866 | 39603 | 5.28 | −3.60457 | 246 |
| 26 | Sex hormone-binding globulin (SHBG) | P04278 | 28876 | 5.32 | −4.0718 | 392 |
| 27 | Fibrinogen gamma chain (FGG) | P02679 | 46823 | 5.54 | 2.03278 | 90 |
| 28 | Apolipoprotein L1 (APOL1) | O14791 | 44004 | 5.60 | −2.34725 | 246 |
| 29 | Complement factor H-related protein 1 (CFHR1) | Q03591 | 38777 | 7.75 | 3.19677 | 180 |
| 30 | Fibrinogen beta chain (FGB) | P02675 | 38081 | 5.84 | 2.24486 | 226 |
| 31 | Sex hormone-binding globulin (SHBG) | P04278 | 28876 | 5.32 | −1000000 | 88 |
| 32 | Serotransferrin (TF) | A0PJA6 | 79280 | 6.81 | −4.3406 | 232 |
| 33 | Serum albumin (ALB) | P02768 | 58513 | 5.96 | −2.53505 | 199 |
| 34 | CFI protein (CFI) | Q8WW88 | 44285 | 8.49 | −2.52761 | 91 |
| 35 | Albumin, isoform CRA_k (ALB) | C9JKR2 | 48568 | 5.97 | −2.16753 | 512 |
| 36 | Ig mu chain C region (IGHM) | P01871 | 50117 | 6.4 | −2.58722 | 257 |
| 37 | Complement factor H-related protein 1 (CFHR1) | Q03591 | 38360 | 7.81 | 1000000 | 244 |
| 38 | Ig kappa chain V-IV region Len | P01625 | 24634 | 6.99 | 1000000 | 101 |
| 39 | Mannose-binding protein C (MBL2) | P11226 | 26530 | 5.22 | −2.04043 | 171 |
| 40 | Complement C4-B (C4B) | P0C0L5 | 48065 | 5.78 | 2.53805 | 221 |
| 41 | Transthyretin (TTR) | P02766 | 12809 | 5.26 | −2.01248 | 266 |
| 42 | Complement C4-B (C4B) | P0C0L5 | 40795 | 5.19 | 2.02316 | 169 |
| 43 | Alpha-1-antitrypsin (SERPINA1) | P01009 | 44280 | 5.37 | −2.69843 | 116 |
| 44 | Ig kappa chain C region (IGKC) | P01834 | 23523 | 5.72 | 3.13516 | 159 |
| 45 | Apolipoprotein M (APOM) | O95445 | 21582 | 5.66 | −4.46038 | 174 |
| 46 | Tetranectin (CLEC3B) | P05452 | 22951 | 5.52 | −1000000 | 295 |
| 47 | Haptoglobin (HP) | P00738 | 42126 | 6.25 | 2.8456 | 172 |
| 48 | Haptoglobin (HP) | P00738 | 42126 | 6.25 | 2.4385 | 109 |
| 49 | Haptoglobin (HP) | P00738 | 42126 | 6.25 | 4.87151 | 176 |
| 50 | Haptoglobin (HP) | P00738 | 42126 | 6.25 | 4.69529 | 193 |
| 51 | Transthyretin (TTR) | P02766 | 12671 | 5.26 | −2.03453 | 236 |
| 52 | Leucine-rich alpha-2-glycoprotein (LRG1) | P02750 | 38382 | 6.45 | 6.51149 | 407 |
CAD/C: ratio of protein in coronary artery dilation caused by Kawasaki disease to control; MW: molecular weight; pI: isoelectric point.
Spot numbers correspond to the numbers marked in Figure 2.
Note: some proteins appeared in 2-DE as multiple spots, probably due to isoforms and/or modifications.
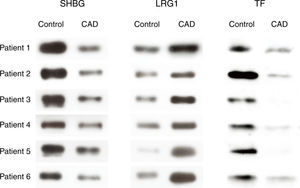
To further confirm the proteomic results, western blot analysis was performed to examine the expression levels of SHBG, LRG1 and TF of serum exosomes from all 12 individuals (six KD patients with CAD and six healthy controls) used in the 2-DE analysis. The validation results were consistent with the 2-DE analysis (Figure 3).
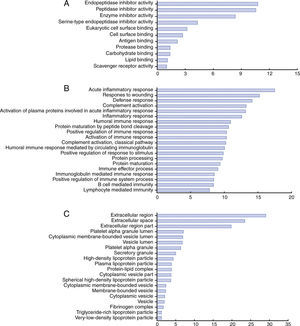
Functional categories and biological interaction networking of identified proteinsTo understand the biological relevance of the changes in protein expression in KD, DAVID software was used to categorize the identified proteins according to their molecular functions, biological processes and protein classes (Figure 4). The molecular functions of the identified proteins were mainly in endopeptidase inhibitor activity, peptidase inhibitor activity, and enzyme inhibitor activity (Figure 4A). These differentially expressed proteins are involved in the acute inflammatory response, response to wounding, defense response, complement activation, humoral immune response and positive regulation of immune response (Figure 4B). As for protein class, most belonged to the extracellular region, platelet alpha granule lumen, cytoplasmic membrane-bounded vesicle lumen and high-density lipoprotein particle classes (Figure 4C).
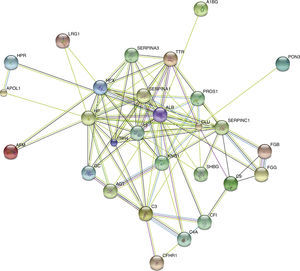
STRING analysis revealed the protein interaction networks in KD patients with CAD (Figure 5). The nodes with highest connectivity were alpha-1-antitrypsin (SERPINA1), serum albumin (ALB), serotransferrin (TF), clusterin (CLU), kininogen-1 (KNG1), and inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4).
DiscussionIn this study, we used 2-DE with MALDI TOF MS to analyze proteomic changes in the serum exosomes of CAD caused by KD. First, we identified 38 differentially expressed proteins in serum exosome samples, providing a proteomic spectrum of KD with CAD. Then, three of these proteins (SHBG, LRG1, and TF) were validated by western blot analysis, confirming that the proteins identified by the proteomic approach were actually differentially expressed proteins. Finally, these differentially expressed proteins were analyzed using a bioinformatics approach. The majority of the differentially expressed proteins were involved in the acute inflammatory response, response to wounding, defense response, complement activation and humoral immune response. KNG1, ITIH4, SERPINC1, SERPINA1, ALB, TTR, TF, and CLU appeared in the center of the functional network intersection and warrant further study. These findings increase our scientific insight and highlight the complexity of the pathogenesis of CAD caused by KD.
ITIH4, one of the inter-alpha-trypsin inhibitor (ITI) family, is a type II acute phase protein involved in inflammatory responses and the host-virus interaction process.9 LRG1 plays an important role in the immune response when the host clears infections. Previous research has shown that LRG1 is involved in important pathological and biological processes, including cell adhesion, signal transduction, and protein-protein interaction.10 SERPINA1 is an effective inhibitor of neutrophil elastase, trypsin, chymotrypsin, plasmin, thrombin, and plasminogen activator. In addition, it is an acute-phase protein and is positively associated with inflammation and necrosis.11 Complement C3 (C3) is an important component of the complement system, involved in the immune response.12 The up-regulation of LRG1 and ITIH4, and down-regulation of C3 and SERPINA1, suggest the immune response to infection may play a role in the pathogenesis of CAD caused by KD.
KNG1 is a protease inhibitor that acts early in the intrinsic pathway of coagulation, in which it is associated with smooth muscle contraction, the inflammatory response, and positive regulation of apoptosis.13 In addition, SERPINC1, fibrinogen gamma chain (FGG), fibrinogen beta chain (FGB), and albumin can also regulate the blood coagulation cascade.14–16 It appears that the regulation of coagulation correlates with the development of CAD caused by KD.
ConclusionIn the present study, we identified 38 proteins that were differentially expressed between KD patients with CAD and normal controls. The identified proteins were classified into different groups according to their molecular functions, biological processes and protein classes based on bioinformatics analysis. The majority of the proteins are involved in the inflammation and coagulation cascades. Although none of the above-mentioned differentially expressed proteins is specifically correlated with CAD caused by KD, the combination of their variations may still provide a useful insight into the pathogenesis of CAD caused by KD.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was supported by Guangzhou City Technology Innovation Foundation of Small and Medium-Sized Enterprises of Technology and Information Bureau (201510010287), Guangzhou City Scientific Research Project Foundation of Technology and Information Bureau (201510010287), the National Clinical Key Specialty Project Foundation.