The natural history and therapeutic interventions for secondary prevention after a cerebrovascular event in patients with patent foramen ovale (PFO) are not yet established. This study aims to assess the safety and efficacy of percutaneous PFO closure in a population of patients with ischemic cerebrovascular disease of unknown etiology.
MethodsThis prospective observational study included patients with a history of cryptogenic transient ischemic attack (TIA) or stroke who underwent percutaneous PFO closure. The effectiveness of the device for the secondary prevention of TIA or stroke was assessed by comparing observed events in the sample with expected events for this clinical setting.
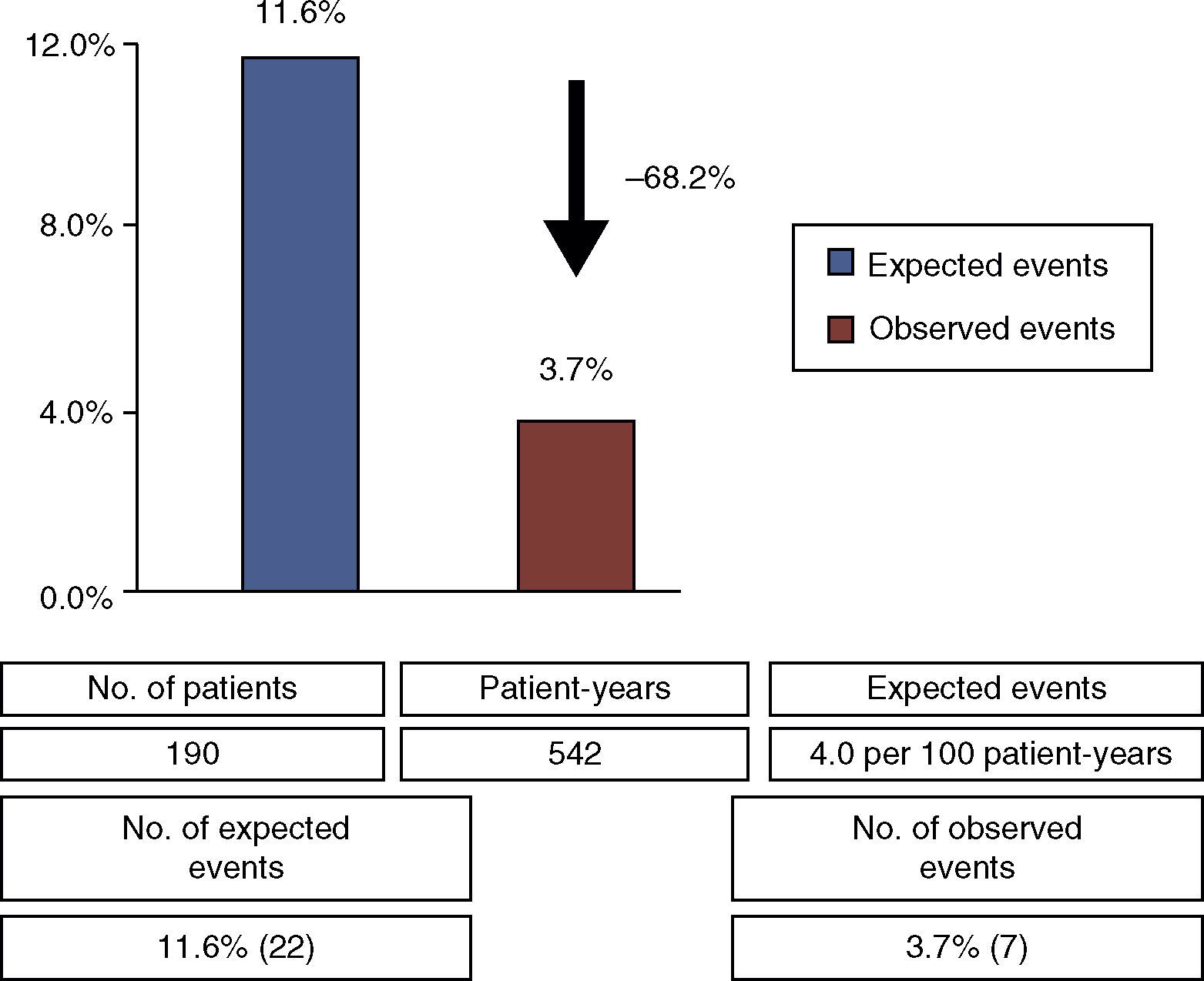
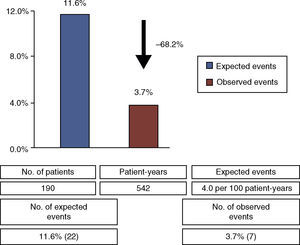
ResultsThe sample included 193 cases of percutaneous PFO closure (age 46.4±13.1 years, 62.2% female) with a mean follow-up of 4.3±2.2 years, corresponding to a total exposure to ischemic events of 542 patient-years. The high-risk characteristics of the PFO were assessed prior to device implantation. There were seven primary endpoint events during follow-up (1.3 per 100 patient-years), corresponding to a relative risk reduction of 68.2% in recurrent TIA or stroke compared to medical therapy alone. The procedure was associated with a low rate of device- or intervention-related complications (1.5%).
ConclusionsIn this long-term registry, percutaneous PFO closure was shown to be a safe and effective therapy for the secondary prevention of cryptogenic stroke or TIA.
A história natural e as intervenções terapêuticas para prevenção secundária, após um evento cerebrovascular em indivíduos com foramen ovale patente (FOP), não estão ainda estabelecidas. Esta investigação visa avaliar a eficácia e a segurança do encerramento de FOP numa população de doentes com doença cerebral isquémica de causa indeterminada.
MétodosEstudo observacional e prospetivo, representativo da região centro de Portugal, que incluiu doentes com antecedentes de acidente isquémico transitório (AIT) ou acidente vascular cerebral (AVC) criptogénico que encerraram FOP por via percutânea. A eficácia do dispositivo na prevenção secundária de AIT/AVC (evento primário) foi avaliada comparando os eventos observados na amostra com os eventos estimados para este contexto clínico.
ResultadosA amostra incluiu 193 casos de encerramento percutâneo de FOP (46,4±13,1 anos, 62,2% do sexo feminino) com um seguimento médio de 4,3±2,2 anos, correspondendo a uma exposição total a eventos isquémicos de 542 doentes/ano. Foram registadas as características anatómicas de risco embólico do FOP previamente à implantação do dispositivo. Observaram-se sete eventos primários (1,3 por 100 doentes/ano), traduzindo uma redução de 68,2% no risco relativo de recorrência de AIT/AVC, em comparação com a terapêutica médica. O procedimento associou-se a uma baixa taxa de complicações relacionadas com a intervenção ou dispositivo (1,5% dos casos).
ConclusõesNeste registo de longa duração o encerramento percutâneo de FOP mostrou-se um procedimento seguro e eficaz na prevenção secundária do AIT/AVC criptogénico.
Despite considerable investment in prevention, stroke remains one of the leading causes of death in Portugal and a major cause of long-term disability.1
Cerebral atherosclerosis is the cause of most ischemic cerebrovascular events, followed by cardioembolism (19% of cases) and carotid artery disease (15%).2 The prevalence of patent foramen ovale (PFO) is around 25% in the general population, and PFO accounts for around 95% of right-to-left shunts. Although PFO is not considered a primary cause of stroke, paradoxical embolism can occur when right atrial pressure exceeds left atrial pressure; this is likely to be the cause of around 40% of cryptogenic strokes.4
In observational studies of patients undergoing antithrombotic therapy, the risk of recurrent stroke or transient ischemic attack (TIA) ranges between 3% and 12% in the first year, with a higher risk in cases with atrial septal aneurysm or large right-to-left shunt. A meta-analysis of 15 major clinical studies of medically treated patients with ischemic cerebrovascular disease of unknown etiology and PFO estimated the rate of recurrent ischemic stroke or TIA at 4.0 events per 100 patient-years and the rate of recurrent ischemic stroke at 1.6 events per 100 patient-years.5
The natural history and therapeutic interventions for secondary prevention after a cerebrovascular event in patients with PFO are not yet established. Percutaneous PFO closure is a therapeutic option for the secondary prevention of cryptogenic stroke, especially in cases of higher risk of paradoxical embolism, when PFO is associated with atrial septal aneurysm or a large shunt.
MethodsStudy design and populationThis prospective observational study was carried out in a university hospital. Informed consent was obtained from all patients prior to enrollment in the study. Inclusion criteria were age over 18 years, history of cryptogenic stroke or TIA, and PFO documented by transesophageal echocardiography. Patients were referred from various hospitals in the Central region of Portugal (Hospitais da Universidade de Coimbra and Hospital Geral do Centro Hospitalar e Universitário in Coimbra, Centro Hospitalar do Baixo Vouga in Aveiro, Hospital Distrital da Figueira da Foz, Centro Hospitalar de Leiria/Pombal, Hospital de São Teotónio in Viseu, Centro Hospitalar da Cova da Beira in Covilhã, and Hospital Amato Lusitano in Castelo Branco).
Ischemic cerebrovascular events were defined as an acute neurological deficit persisting for over 24 hours, or less than 24 hours but associated with evidence of cerebral infarction on magnetic resonance imaging or computed tomography. Known causes of stroke including cardioembolism, carotid artery disease, lacunar cerebral infarction due to small vessel disease, and documented hypercoagulable states, such as the presence of anticardiolipin antibodies or lupus anticoagulant or hyperhomocysteinemia, were excluded.
PFO was defined by transesophageal echocardiography as evidence of passage of microbubbles from right to left atrium within three cardiac cycles following injection of agitated saline at rest and with the Valsalva maneuver. The shunt was classified as grade 1 (small: 1–20 microbubbles) or 2 (large: >20 microbubbles).6 Atrial septal aneurysm was defined as a mobile protrusion of the septum primum tissue into the atrium measuring ≥10 mm.6 The clinical characteristics of the study population included cardioembolic risk factors according to the CHA2DS2-VASc risk score,7 which was calculated without taking into account previous cerebrovascular events, since this was common to all patients.
Percutaneous PFO closure was performed using one of the following devices: Amplatzer™ PFO Occluder, Premere™ PFO Closure System, Occlutech Figulla™ occluder, Solysafe™ Septal Occluder or Gore Helex™ Septal Occluder, under fluoroscopic and intracardiac or transesophageal echocardiographic guidance.6 Procedural success was defined as successful implantation of the closure device.
Postprocedural follow-up was by transthoracic echocardiography at hospital discharge, at 1, 3, 6 and 12 months and thereafter annually. Patients were prescribed aspirin and clopidogrel for 1–3 months following the procedure, followed by single antiplatelet therapy or another antithrombotic regimen as prescribed by their attending physician, in most cases a neurologist.
Periprocedural adverse eventsPeriprocedural adverse events were defined as those occurring within seven days of implantation or before hospital discharge based on the criteria of the Valve Academic Research Consortium-2 consensus document,8 including death, myocardial infarction, stroke, TIA, systemic embolization, device embolization, pericardial effusion or cardiac tamponade, or major bleeding requiring surgery or transfusion.
Clinical follow-upThe primary endpoint event was defined as new stroke or TIA during follow-up, based on symptoms, neurological exams and relevant imaging studies.
In view of the absence of a control group, device effectiveness for secondary prevention of stroke or TIA was tested by comparing the rate of cerebrovascular events in the study population with the rate in a control group in the literature.5 The total number of cerebrovascular events in the study population was divided by the number of patient-years and multiplied by 100 to give the annual incidence of thromboembolism. Relative risk reduction was calculated as (expected event rate minus observed event rate) divided by expected event rate, and the number needed to treat (NNT) to prove benefit from PFO closure as the inverse of the absolute reduction in new events (1 divided by observed event rate minus expected event rate).
Secondary endpoint events were defined as all-cause death during follow-up, new stroke during follow-up, and peridevice leak in the atrial septum more than three months after the procedure. Complete PFO closure was defined as absence of interatrial and peridevice flow during follow-up. Peridevice leaks were assessed by color Doppler and classified as small (1–3 mm) or large (>3 mm).6 Transthoracic echocardiographic study to detect intracavitary thrombus, device-associated or in the atrial chamber, was performed during follow-up.
Statistical analysisContinuous variables were presented as means ± standard deviation and categorical variables as relative frequencies. The statistical analysis was performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
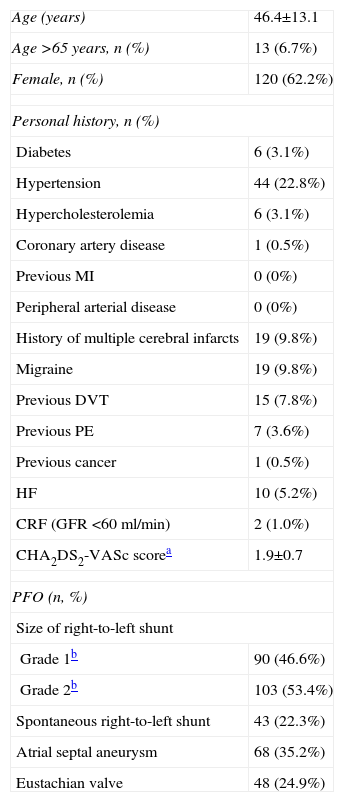
ResultsStudy populationThe sample included 193 cases of percutaneous PFO closure between January 2005 and December 2013. The baseline characteristics are summarized in Table 1. Mean age was 46.4±13.1 years, and only 13 patients (6.7%) were aged over 65; most were female (62.2%). The incidence of comorbidities was low, hypertension being the most frequent cardiovascular risk factor (n=44, 22.8%). The mean CHA2DS2-VASc score was 1.7±0.6, and 46 patients (23.8%) had a score of 0; most of the points in the score were from hypertension and female gender. On the basis of the mean CHA2DS2-VASc score, the expected annual risk for a new cerebrovascular event if undiagnosed AF had been responsible for the initial event would be 1.9%/year.
Baseline characteristics of the study population (n=193).
| Age (years) | 46.4±13.1 |
| Age >65 years, n (%) | 13 (6.7%) |
| Female, n (%) | 120 (62.2%) |
| Personal history, n (%) | |
| Diabetes | 6 (3.1%) |
| Hypertension | 44 (22.8%) |
| Hypercholesterolemia | 6 (3.1%) |
| Coronary artery disease | 1 (0.5%) |
| Previous MI | 0 (0%) |
| Peripheral arterial disease | 0 (0%) |
| History of multiple cerebral infarcts | 19 (9.8%) |
| Migraine | 19 (9.8%) |
| Previous DVT | 15 (7.8%) |
| Previous PE | 7 (3.6%) |
| Previous cancer | 1 (0.5%) |
| HF | 10 (5.2%) |
| CRF (GFR <60 ml/min) | 2 (1.0%) |
| CHA2DS2-VASc scorea | 1.9±0.7 |
| PFO (n, %) | |
| Size of right-to-left shunt | |
| Grade 1b | 90 (46.6%) |
| Grade 2b | 103 (53.4%) |
| Spontaneous right-to-left shunt | 43 (22.3%) |
| Atrial septal aneurysm | 68 (35.2%) |
| Eustachian valve | 48 (24.9%) |
CRF: chronic renal failure; DVT: deep vein thrombosis; GFR: glomerular filtration rate; HF: heart failure; MI: myocardial infarction; PE: pulmonary embolism; PFO: patent foramen ovale.
The anatomical characteristics of the PFO associated with embolic risk were documented by preprocedural transesophageal echocardiography (Table 1). In more than half of cases (n=103, 53.4%) there was a grade 2 right-to-left shunt, and the incidence of other high-risk characteristics was high, including atrial septal aneurysm (n=68, 35.2%), spontaneous right-to-left shunt (n=43, 22.3%) and a prominent Eustachian valve directing blood flow from the vena cava to the PFO (n=48, 24.9%); several patients presented more than one of these characteristics.
ProcedureThe devices used were the Amplatzer™ PFO Occluder (n=105, 54.4%), Premere™ PFO Closure System (n=61, 31.6%), Occlutech Figulla™ occluder (n=21, 11.0%), Solysafe™ Septal Occluder (n=5, 2.5%), and Gore Helex™ Septal Occluder(n=1, 0.5%). Mean occluder size was 24 mm (9–35).
One adverse event occurred during the procedure (0.5%), an access-related femoral pseudoaneurysm, treated by mechanical compression. There were no other periprocedural adverse events. There were also no cases of gas embolism during implantation or consequent need for treatment of neurological sequelae.
Clinical follow-upFollow-up was achieved in 190 patients (98.4%), for a mean of 4.3±2.2 years (0–9.2). Total exposure to ischemic events was 197 710 days, corresponding to 542 patient-years. Three months after the procedure, 39 patients (20.5%) were not taking antithrombotic medication, 145 (74.7%) were under single antiplatelet therapy and nine (4.7%) were under oral anticoagulation alone.
There were seven primary endpoint events (3.7% of the 190 patients who completed follow-up), all non-fatal stroke or TIA, with a time to event of 3.7±2.1 years, a rate of 1.3 events per 100 patient-years. Considering the expected rate of recurrent cryptogenic ischemic stroke or TIA in medically treated patients with PFO (4.0 events per 100 patient-years5), percutaneous PFO closure led to a relative risk reduction of 68.2% (Figure 1), with an NNT of 13.
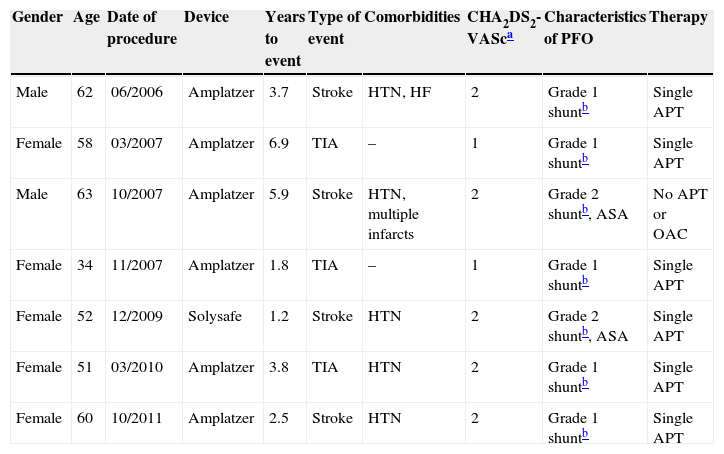
Table 2 shows the characteristics of patients with primary endpoint events. Nearly all were over 50 years old and with few comorbidities; CHA2DS2-VASc scores were between 1 and 2 and none was under oral anticoagulation. In most cases there was a small right-to-left shunt and only two cases presented high-risk PFO features (septal aneurysm).
Characteristics of patients in the sample with primary endpoint events.
| Gender | Age | Date of procedure | Device | Years to event | Type of event | Comorbidities | CHA2DS2-VASca | Characteristics of PFO | Therapy |
|---|---|---|---|---|---|---|---|---|---|
| Male | 62 | 06/2006 | Amplatzer | 3.7 | Stroke | HTN, HF | 2 | Grade 1 shuntb | Single APT |
| Female | 58 | 03/2007 | Amplatzer | 6.9 | TIA | – | 1 | Grade 1 shuntb | Single APT |
| Male | 63 | 10/2007 | Amplatzer | 5.9 | Stroke | HTN, multiple infarcts | 2 | Grade 2 shuntb, ASA | No APT or OAC |
| Female | 34 | 11/2007 | Amplatzer | 1.8 | TIA | – | 1 | Grade 1 shuntb | Single APT |
| Female | 52 | 12/2009 | Solysafe | 1.2 | Stroke | HTN | 2 | Grade 2 shuntb, ASA | Single APT |
| Female | 51 | 03/2010 | Amplatzer | 3.8 | TIA | HTN | 2 | Grade 1 shuntb | Single APT |
| Female | 60 | 10/2011 | Amplatzer | 2.5 | Stroke | HTN | 2 | Grade 1 shuntb | Single APT |
APT: antiplatelet therapy; ASA: atrial septal aneurysm; HF: heart failure; HTN: hypertension; OAC: oral anticoagulation; PFO: patent foramen ovale.
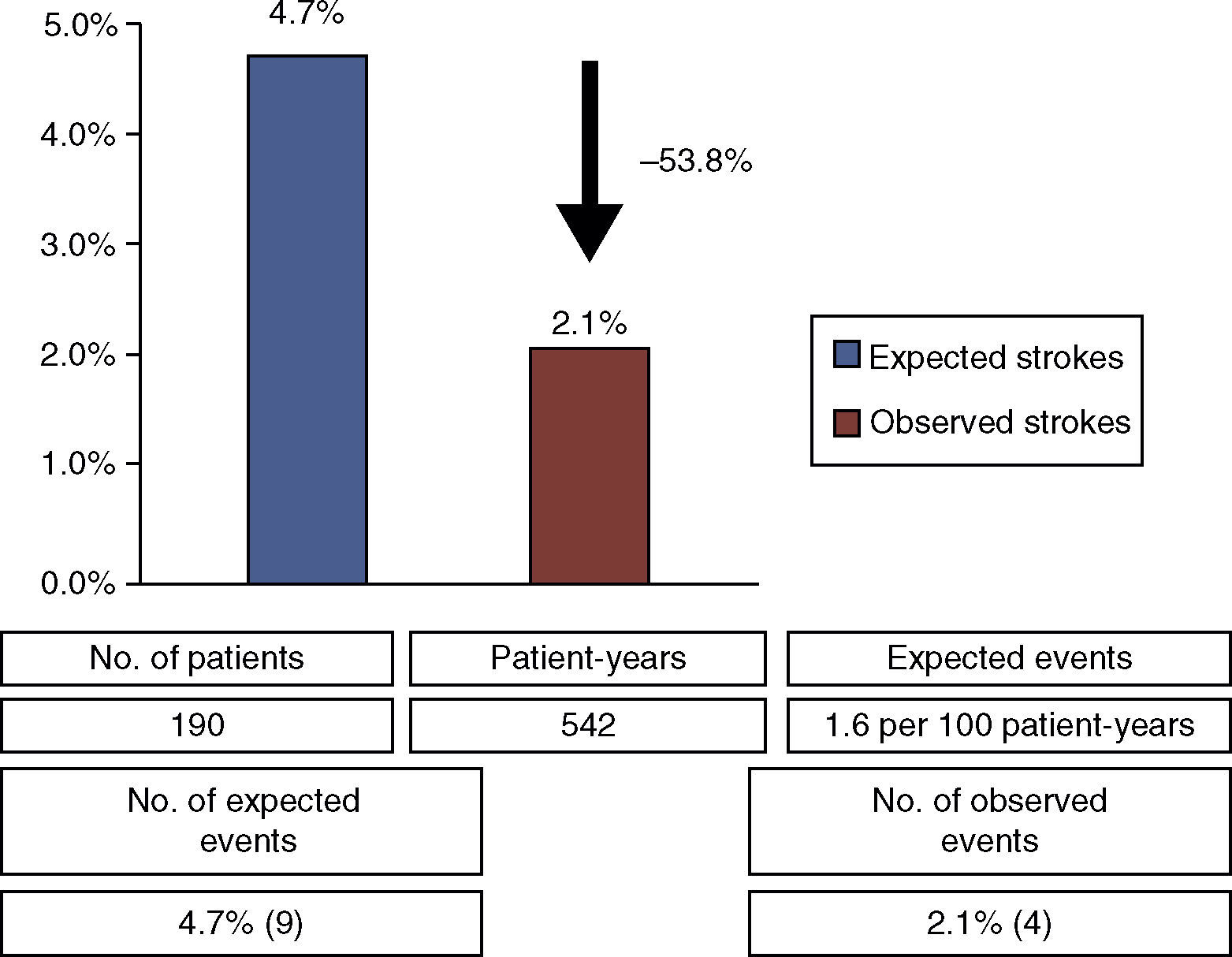
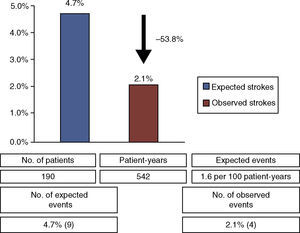
Regarding secondary endpoints, there were four cases identified as new ischemic stroke on imaging studies (57.1% of cerebrovascular events during follow-up). The stroke rate was 0.7 events per 100 patient-years, corresponding to a relative risk reduction of 53.8% compared to the expected rate of isolated stroke in this clinical setting (1.6 events per 100 patient-years5) (Figure 2). There was one non-cardiovascular death, from septic shock (n=1, 0.5%). Echocardiographic follow-up studies revealed two cases (1.1%) of peridevice leak more than three months after the procedure, both small (1–3 mm). No device-related or atrial thrombi were detected.
DiscussionThe results of this registry, representative of the Central region of Portugal, in prevention of new ischemic cerebrovascular events were compared with the estimated risk in the literature5 for this clinical setting, since it lacks a control group. A relative risk reduction of 68.2% in recurrent TIA or stroke and of 53.8% in isolated stroke was observed in patients who underwent percutaneous PFO closure compared to data on medical therapy alone. Percutaneous PFO closure was shown to be safe, with a rate of device- or intervention-related complications of 1.5% (access-related femoral pseudoaneurysm and peridevice leak). The long follow-up, corresponding to a total exposure to ischemic events of 542 patient-years, considerably enhances the results of the study.
The etiology of stroke or TIA cannot be established in 40% of ischemic cerebrovascular events even after exhaustive diagnostic investigation, particularly in younger patients.3 Such events are often classified as cryptogenic even in the presence of PFO, the most likely triggering mechanism. Although the association with PFO has been repeatedly confirmed, paradoxical cerebral embolism is rare, and is usually assumed rather than proven. This is also the case for stroke or TIA attributed to atrial fibrillation or to atherosclerotic disease of the ascending aorta or carotid arteries, or associated with myocardial infarction.
The natural history following cryptogenic stroke or TIA has yet to be established, particularly regarding the risk of recurrence and the value of interventions for secondary prevention. Embolic risk stratification in these patients should include high-risk PFO features, as well as the standard embolic risk factors as assessed by the CHA2DS2-VASc score.10 The study population consisted of relatively young adults (mean age 46 years), with a low incidence of risk factors for cardioembolism or other comorbidities, common causes of stroke or TIA having been excluded; the population was thus at low cardiovascular risk and ischemic cerebrovascular events, particularly recurrent ones, were considered unlikely. Such cases are often attributed to silent atrial fibrillation, but in our population with low CHA2DS2-VASc scores (mean score 1.7±0.6 and 23.5% of patients with a score of 0), this etiology is improbable. The low mortality seen in the study (0.5%) is a hard endpoint that indicates a population with very low cardiovascular risk. Conventional thromboembolic risk scores were not designed to be applied in secondary prevention of ischemic cerebrovascular disease, and no risk algorithm has been recommended to guide therapeutic decision-making in these patients. The most recent advance comes from the investigators of the RoPE (Risk of Paradoxical Embolism) study, who developed a risk model that uses only clinical variables to estimate the risk of a new ischemic cerebrovascular event in patients with PFO.11 The risk is also related to the morphological and functional characteristics of the PFO, including the grade of right-to-left shunt and the presence of atrial septal aneurysm.12 The higher embolic risk associated with these features has been established by several observational studies and was recently confirmed by subgroup analysis of the RESPECT trial9 that showed greater benefit from closure compared to medical therapy alone in the presence of a substantial shunt (hazard ratio [HR] 0.18, 95% confidence interval [CI] 0.04–0.81) and atrial septal aneurysm (HR 0.19, 95% CI 0.04–0.87). However, neither the RoPE score nor the presence of these high-risk features can yet be used to justify percutaneous PFO closure according to the international guidelines.10
Patients with a history of cryptogenic stroke or TIA have conventionally been prescribed antithrombotic therapy, although it is not established whether oral anticoagulation is superior to antiplatelet therapy.10 Medical therapy is associated with significant adverse effects, particularly in the long term, 1.5–2.2 major bleeds occurring per 100 patient-years, especially with chronic oral anticoagulation, as reported in the main studies on medical therapy for secondary prevention, the Warfarin-Aspirin Recurrent Stroke Study (WARSS)12 and the Patent Foramen Ovale in Cryptogenic Stroke Study (PICSS)13. Since the rate of recurrent stroke in this population is low (1.6 events per 100 patient-years5), the benefit of antithrombotic therapy is debatable. The long-term safety of any preventive therapy is a major concern when the natural history of the condition is of low ischemic risk.
Although non-randomized studies are consistently in favor of PFO closure, the main randomized trials9,14 did not prove superiority over medical treatment. The low statistical power of the results was due to the small study samples, the fact that the rate of ischemic events was lower than predicted, and the need for a much longer follow-up. Overall, the results suggest that device closure is as clinically effective as anticoagulation and probably superior to antiplatelet therapy,9,12 and is a valid alternative particularly in cases of recurrent ischemic events due to failure of or contraindication to antithrombotic therapy or to patients’ difficulty in adhering to medication. Another finding in favor of PFO closure in the RESPECT trial9 was larger cerebral infarcts in the medical therapy group than in the closure group.
Data from this registry reveal the characteristics of patients who suffered a new ischemic cerebrovascular event (Table 2). Our results highlight the importance of patient selection for PFO closure. Older patients should be referred, age being one of the main risk factors for ischemic events, but on the other hand, the absence of high-risk PFO features indicates a lower likelihood of paradoxical embolism as the cause of the index event.
This study has certain limitations. It is an observational study without independent verification of the results. However, the patients had been diagnosed, and known etiologies of stroke and TIA had been excluded, by neurologists, who also independently confirmed the occurrence of new events. There was no control group, and therefore the efficacy of PFO closure for secondary prevention of stroke was not assessed directly. Finally, the sample consisted of patients at high risk for paradoxical embolism, rather than being an all-comer population with ischemic cerebrovascular disease of undetermined cause.
ConclusionsIn this observational study with long-term follow-up, percutaneous PFO closure was shown to be a safe and effective therapy in the secondary prevention of cryptogenic stroke or TIA. Confirmation of this benefit in future studies may lead to changes in antithrombotic therapy for these patients, reducing the risk of bleeding events.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Paiva L, Dinis P, Providência R, et al. Encerramento percutâneo de foramen ovale patente – registo da prevenção da embolia cerebral paradoxal. Rev Port Cardiol. 2015;34:151–157.