In non-valvular atrial fibrillation 90% of thrombi originate in the left atrial appendage (LAA). Percutaneous LAA closure has been shown to be non-inferior to warfarin for prevention of thromboembolism.
ObjectiveTo evaluate the initial experience of a single center in percutaneous LAA closure in patients with high thromboembolic risk and in whom oral anticoagulation was impractical or contraindicated or had failed.
MethodsPatients with non-valvular atrial fibrillation and CHADS2 score ≥2 in whom oral anticoagulation was impractical or contraindicated or had failed underwent percutaneous LAA closure according to the standard technique. After the procedure, dual antiplatelet therapy was maintained for one month, followed by single antiplatelet therapy indefinitely. Patients were followed by clinical assessment and transthoracic and transesophageal echocardiography.
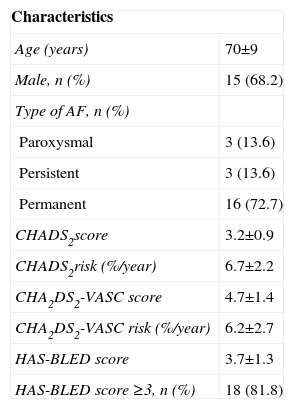
ResultsThe procedure was performed in 22 of the 23 selected patients (95.7%), mean age 70±9 years, CHADS2 score 3.2±0.9 and CHA2DS2-VASC score 4.7±1.4. Intraprocedural device replacement was necessary only in the first patient, due to oversizing. The following periprocedural complications were observed: one femoral pseudoaneurysm, three femoral hematomas and two minor oropharyngeal bleeds, resolved by local hemostatic measures. During a 12±8 month follow-up a mild peri-device flow and a thrombus adhering to the device, resolved under with enoxaparin therapy, were identified. The rate of transient ischemic attack (TIA)/stroke was lower than expected according to the CHADS2 score (0 vs. 6.7±2.2%).
ConclusionsIn our initial experience, this procedure proved to be a feasible, safe and effective alternative for atrial fibrillation patients in whom oral anticoagulation is not an option. Only relatively minor complications were observed, with a lower than expected TIA/stroke rate.
Na fibrilhação auricular não valvular, 90% dos trombos originam-se no apêndice auricular esquerdo. O seu encerramento percutâneo mostrou ser não inferior à varfarina na profilaxia do tromboembolismo.
ObjetivoAvaliar a experiência inicial de um centro no encerramento percutâneo do apêndice auricular esquerdo em doentes com elevado risco tromboembólico sem possibilidade, com contraindicação ou falência da anticoagulação oral.
MétodosDoentes com fibrilhação auricular não valvular, score CHADS2 ≥ 2 sem possibilidade, com contraindicação ou falência da anticoagulação oral foram submetidos a encerramento percutâneo do apêndice auricular esquerdo de acordo com a técnica padrão. Após implantação, foi mantida dupla antiagregação durante um mês e simples indefinidamente. Realizado seguimento com avaliação clínica, ecocardiografia transtorácica e transesofágica.
ResultadosO procedimento foi conseguido em 22 dos 23 doentes selecionados (95,7%): 70±9 anos, score CHADS2 de 3,2±0,9 e CHA2DS2-VASC de 4,7±1,4. Apenas no primeiro doente o dispositivo foi substituído por sobredimensionamento. Foram observadas as seguintes complicações periprocedimento: um pseudoaneurisma femoral, 3 hematomas femorais e 2 hemorragias da orofaringe, resolvidos com medidas locais. Durante o seguimento de 12±8 meses foram identificados um fluxo peridispositivo ligeiro e um trombo aderente ao dispositivo - que resolveu sob enoxaparina. A taxa de AVC/AIT foi inferior à esperada com base no score CHADS2 (0 versus 6,7±2,2%).
ConclusõesNa nossa experiência inicial este procedimento mostrou ser uma alternativa exequível, segura e eficaz em doentes com fibrilhação auricular para os quais a anticoagulação oral não é uma opção. Foram identificadas complicações de baixa severidade, com uma taxa de AVC/AIT inferior à esperada.
Atrial fibrillation (AF) is a common cardiac arrhythmia in clinical practice. Its prevalence in Portugal is 2.5% in those aged 40 or over according to the FAMA study.1 The figure in the general population is 1–2%, rising with age; prevalence has increased significantly over time and is predicted to double in the next 50 years.2
AF is associated with high morbidity and mortality due to its thromboembolic potential; it doubles the risk of death independently of other factors and increases the risk of stroke five-fold compared to individuals of the same age in sinus rhythm.2,3 The large size of the thrombi that cause these strokes means that their consequences tend to be more severe than from other sources of cerebral thrombi, are often fatal or severely disabling, and are more likely to recur.2–6
The thromboembolic potential is similar for all forms of AF, including paroxysmal.1–3 The method for assessment of stroke risk in AF recommended by the European Society of Cardiology is the CHADS2 score, which is based on a point system in which 2 points are assigned for a history of stroke or transient ischemic attack (TIA) and 1 point each is assigned for age >75 years, a history of hypertension, diabetes, or recent cardiac failure. A CHADS2 score ≥2 corresponds to a stroke risk of ≥4%/year and is an indication for oral anticoagulation (OAC). For a CHADS2 score of 0 or 1 the decision on whether to institute OAC should be reassessed considering ‘clinically relevant non-major’ risk factors using the CHA2DS2-VASc score, which assigns 2 points for a history of stroke or TIA, or age ≥75; and 1 point each for age 65–74 years, a history of hypertension, diabetes, recent cardiac failure, vascular disease (myocardial infarction, complex aortic plaque, and peripheral arterial disease), and female gender. OAC is indicated for a CHA2DS2-VASc score of ≥2 (annual stroke risk ≥2.2%).2
Antithrombotic therapy has been shown to reduce mortality and stroke in AF.2 Warfarin is the first line treatment for thromboembolic prevention, reducing relative risk of stroke by 60–73% for an international normalized ratio (INR) of 2–3,2–9 clearly superior to aspirin, which achieved a reduction of only 20%.2,7 There are concerns over bleeding complications with warfarin use, the most feared of which is intracranial bleeding, for which the risk rises for INR over 3.5.2–7 Assessment of the individual patient's bleeding risk is thus an essential step in the decision to prescribe anticoagulation, and for this the ESC guidelines recommend the HAS-BLED score (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly (>65), drugs/alcohol concomitantly), in which a score of ≥3 indicates ‘high risk’, and some caution and regular monitoring of the patient is needed following the initiation of antithrombotic therapy.2
However, warfarin is contraindicated in 14–44% of AF patients at risk of stroke.10 Even among eligible patients, only 54% are anticoagulated, for a variety of reasons, including the need for frequent laboratory testing and for the patient's cooperation, and clinicians’ wariness.4,5 Furthermore, anticoagulated patients present INR values within the therapeutic window in only 50–68% of tests.9
These obstacles to warfarin therapy have led to a search for alternatives for thromboembolic prevention in AF. The new anticoagulants are divided into two main classes: direct thrombin inhibitors such as dabigatran, and factor Xa inhibitors such as rivaroxaban, apixaban, edoxaban and betrixaban. They all have advantages over warfarin including a wider therapeutic window, fewer interactions with foods, and no need for laboratory monitoring.2 Dabigatran (the RE-LY study11), rivaroxaban (ROCKETAF12) and apixaban (ARISTOTLE13) have demonstrated non-inferiority to warfarin in thromboembolic prevention in AF, and the first two have been approved by the US Food and Drug Administration (FDA) for this purpose.14 The latest guidelines for the management of atrial fibrillation of the European Society of Cardiology consider dabigatran an alternative to warfarin in AF patients with indication for OAC.2 The latest Canadian Cardiovascular Society guidelines also recommend the use of dabigatran rather than warfarin in these patients.10
However, these drugs are expensive for chronic therapy, carry a significant bleeding risk, and do not have an established antidote, all which are obstacles to their use in many patients. Studies on these new anticoagulants have also shown significant rates of discontinuation of therapy, mainly due to intolerance or adverse effects, reaching 25.3% in patients taking apixaban (vs. 27.5% for those taking warfarin) in the ARISTOTLE trial,13 but higher than seen for warfarin in the RE-LY11 (21% for dabigatran vs. 17%) and ROCKET-AF12 (23.7% for rivaroxaban vs. 22.2%) trials.
At the same time, all these drugs are contraindicated in patients with a history of hemorrhagic stroke, which, when taken together with their high thromboembolic risk, constitutes a dilemma in terms of stroke prevention.
The left atrial appendage (LAA) has been identified by autopsy studies, transesophageal echocardiography (TEE) and direct intraoperative inspection as the most common site of intracardiac thrombi in AF, accounting for 58% of sites in valvular AF and 90–98% in non-valvular AF.15–19
In the light of these facts, percutaneous closure of the LAA was seen as an alternative to pharmacological anticoagulation to prevent thromboembolism in AF. Three different types of device have been developed for percutaneous LAA closure, the feasibility of which has been demonstrated in numerous trials.4,5,9,19,20
The PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) system (ev3 Inc., Plymouth, MN)21 was the first device to be designed and used for percutaneous LAA closure. It consisted of a self-expanding nitinol cage coated with a polytetrafluoroethylene membrane designed both to occlude LAA flow and to allow tissue incorporation into the device, and three rows of anchors to attach the device to the LAA orifice. Several series were published confirming the feasibility and efficacy of this device for thromboembolic prevention in AF, but reports of infrequent but serious complications led to the manufacturer withdrawing the device from the market.19,21
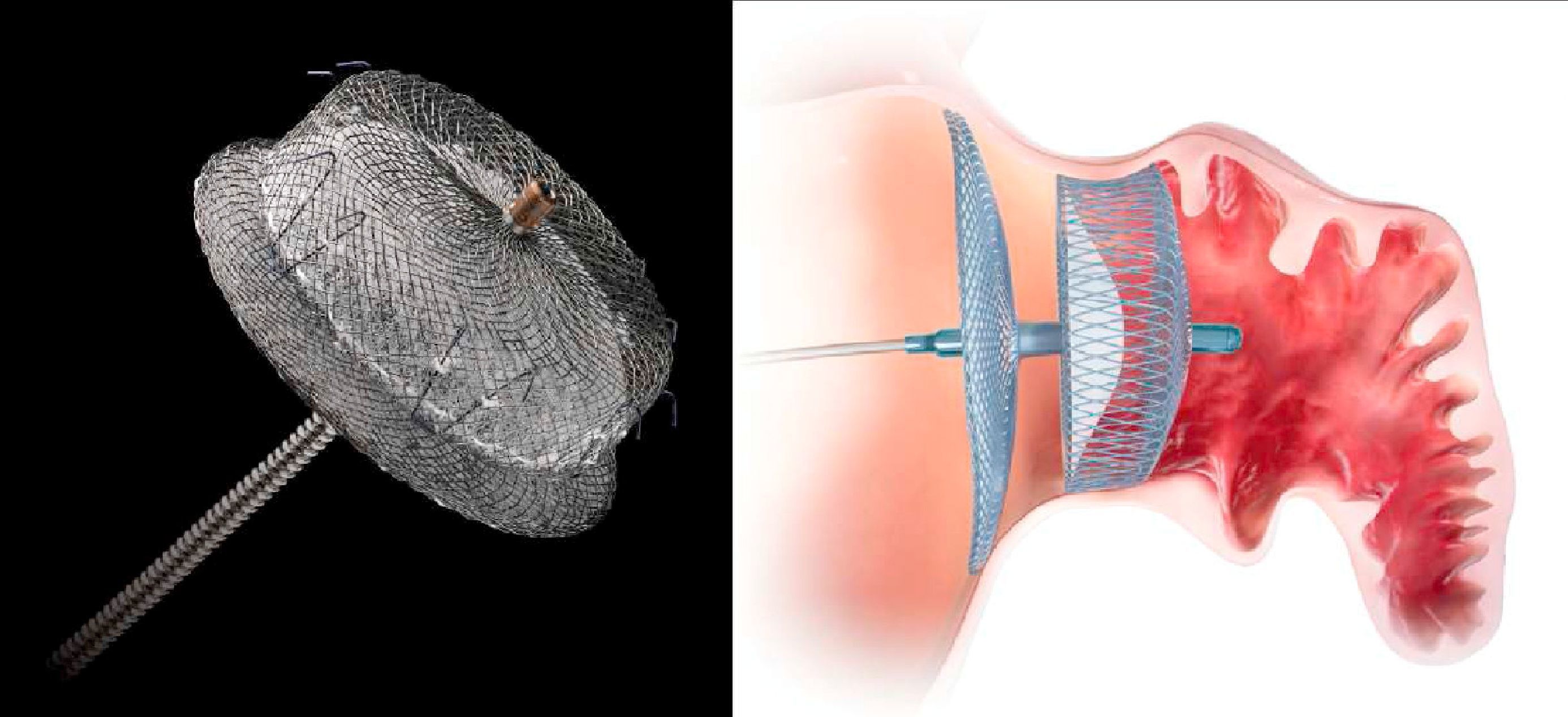
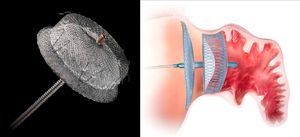
The second device developed for LAA closure is the Amplatzer Cardiac Plug (ACP) (St. Jude Medical, Plymouth, MN),22 with a self-expanding nitinol frame covered in a polyester patch, consisting of a lobe and a disc connected by a central waist. Hooks on the lobe fix the device, while the disc seals the LAA orifice (Figure 1). It is available in eight lobe sizes from 16 to 30 mm at 2-mm intervals.22 Its feasibility and safety have been demonstrated in various series, all of which showed a stroke/TIA rate lower than that expected on the basis of the CHADS2 score. After the procedure, patients in these series were medicated with clopidogrel for 1–3 months and aspirin for three months or indefinitely. However, concerns subsequently surfaced about thrombus formation on the device, prompting the manufacturer to issue a Field Safety Notice updating the instructions for use and recommending aspirin for six months post-implant, leaving the decision to continue this regimen thereafter at the discretion of the physician, and recommending clopidogrel or an alternate antiplatelet, with prescription following routine standard of care.19
The prospective randomized Amplatzer Cardiac Plug Clinical Trial is currently under way, comparing the efficacy of the ACP with warfarin in patients with AF and CHADS2 score ≥2, and without contraindication for OAC.38
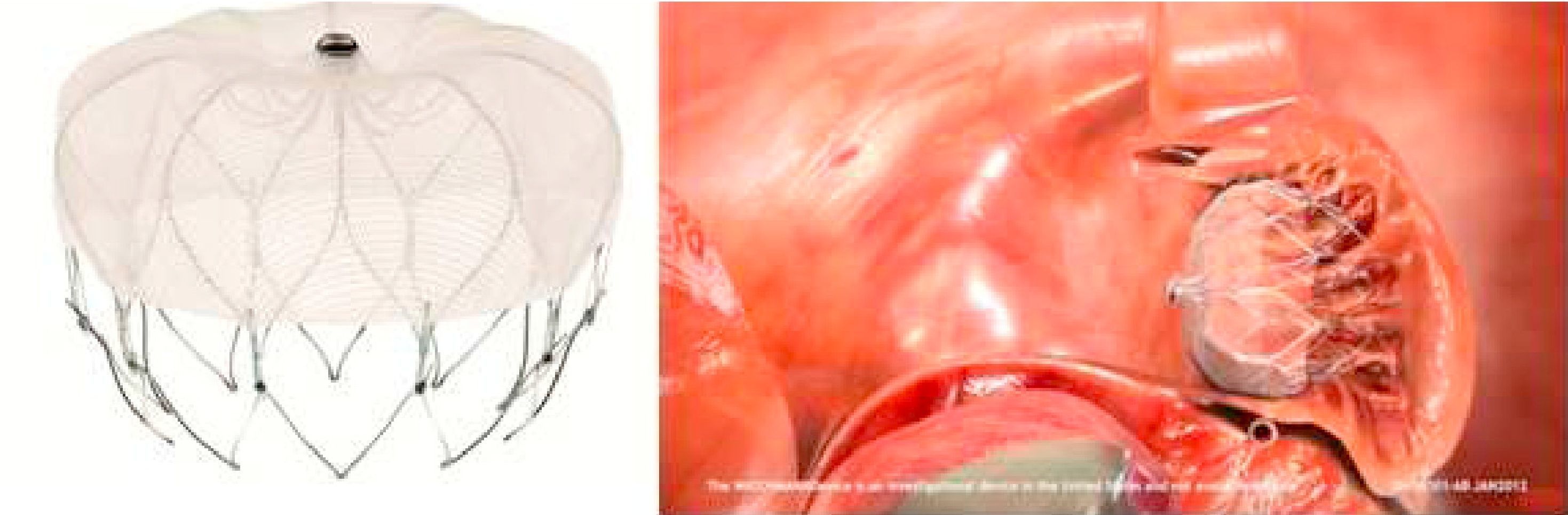
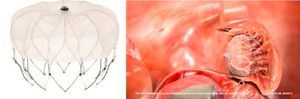
The Watchman device9 (Boston Scientific, Plymouth, MN) was also designed specifically for percutaneous LAA closure. It consists of a self-expanding nitinol frame with fixation barbs around its perimeter and a porous polyester membrane only on the LA-facing surface (Figure 2). It is available in five diameters from 21 to 33 mm at 3-mm intervals.9 Its feasibility and safety have also been demonstrated. Initial post-procedural medication specified warfarin for 45 days and aspirin indefinitely, which was later modified to include clopidogrel between 45 days and the six-month follow-up.19 The non-inferiority of percutaneous LAA closure with the Watchman device compared to warfarin was established by the randomized PROTECT AF trial,8,19 which included patients with non-valvular AF and CHADS2 score of ≥1. As more patients underwent treatment with this device and with a longer follow-up in the CAP registry,23 fears concerning the safety of the technique began to recede; it became clear that the complications were largely procedure-related, decreased in frequency with greater operator experience, and led to less significant disability than those related to warfarin therapy, which accumulate linearly over time.19,23
The invasive nature of percutaneous LAA closure means that in theory the best risk/benefit ratio will be in patients with greater thromboembolic risk and contraindication to anticoagulation therapy. However, the PROTECT AF trial8 included patients with CHADS2 score of ≥1, and the applicability of its results to those at higher risk is questionable. Furthermore, patients in that trial were medicated with warfarin for 45 days after the procedure, which also limits its applicability to those with contraindication to OAC.
Reports of percutaneous LAA closure combined with antiplatelet therapy (aspirin with or without clopidogrel, both for varying periods) with no initial period of anticoagulation post-implantation have been published in series on the PLAATO device (which detected a low rate of thromboembolic events),19 on the ACP device (which reported some cases of device-related thrombi),19 and on the Watchman device in the ASA Plavix Feasibility Study With WATCHMAN Left Atrial Appendage Closure Technology (ASAP) prospective non-randomized registry.24 The latter included 150 patients with non-valvular AF, CHADS2 score of ≥1 and contraindication to warfarin who took clopidogrel for six months and aspirin indefinitely; the authors concluded that implantation of the Watchman was safe and effective without temporary warfarin therapy.25
The aim of the present study was to evaluate the initial experience of a single center in percutaneous LAA closure in patients with high thromboembolic risk and in whom OAC was impractical or contraindicated or had failed, between May 2010 and June 2012.
MethodsPatient selectionBetween May 2010 and June 2012, 23 patients with non-valvular AF of any type (permanent, persistent or paroxysmal) and high thromboembolic risk (CHADS2 score ≥2), in whom OAC was impractical or contraindicated or had failed, were selected for percutaneous LAA closure in our center.
OAC was considered impractical when medication with vitamin K antagonists was not an option, due to labile INR or inability to monitor INR.
Contraindication to OAC was defined as the presence of a history of cerebral bleeding, major bleeding under OAC or antiplatelet therapy, or bleeding dyscrasia. Anticoagulation therapy was considered to have failed when an embolic event had occurred and a thrombus had been detected in the LAA despite anticoagulation with warfarin and therapeutic INR levels.
A CHADS2 score ≥2 was chosen as an inclusion criterion because this is the level of risk at which thromboembolic prevention with OAC is indicated. Since it was intended to include patients in whom warfarin therapy was impractical, it was considered that this criterion would select those who in theory would benefit most from the nonpharmacological alternative under analysis.
Exclusion criteria were the presence of congenital heart disease, valve disease, known or suspected hypercoagulable state, pregnancy, allergy to nickel, active endocarditis or possible source of bacteremia, mechanical prosthetic valve, pacemaker leads or implantable cardioverter-defibrillator in the cardiac chambers, intracardiac thrombus, or an LAA too small for percutaneous closure using the available devices.
In order to assess the latter two criteria, TEE was performed in all patients before the procedure to measure the LAA orifice and to exclude thrombi, particularly in the LAA.
Device implantation and follow-up protocolSelected patients were admitted the day before the procedure and medicated with dual antiplatelet therapy (DAT) (aspirin 100 mg i.d. and clopidogrel 75 mg i.d.), subcutaneous enoxaparin 1 mg/kg/h every 12 hours (except for the last dose prior to the procedure), and saline infusion 1 ml/kg/h begun 12 hours before the procedure and continued for 24 hours afterwards to prevent contrast nephropathy and to maintain intravascular and LAA volume. Cefuroxime 750 mg was administered intravenously one hour before the procedure for bacterial endocarditis prophylaxis.
All procedures were performed under deep sedation or general anesthesia with an anesthetist in attendance. Access was via a femoral route and implantation was guided by fluoroscopy and TEE. During the procedure an intravenous heparin bolus was administered to achieve partial activated thromboplastin time of ≥250 s.
Two types of closure device were used, the ACP and the Watchman. All were implanted by the same operator.
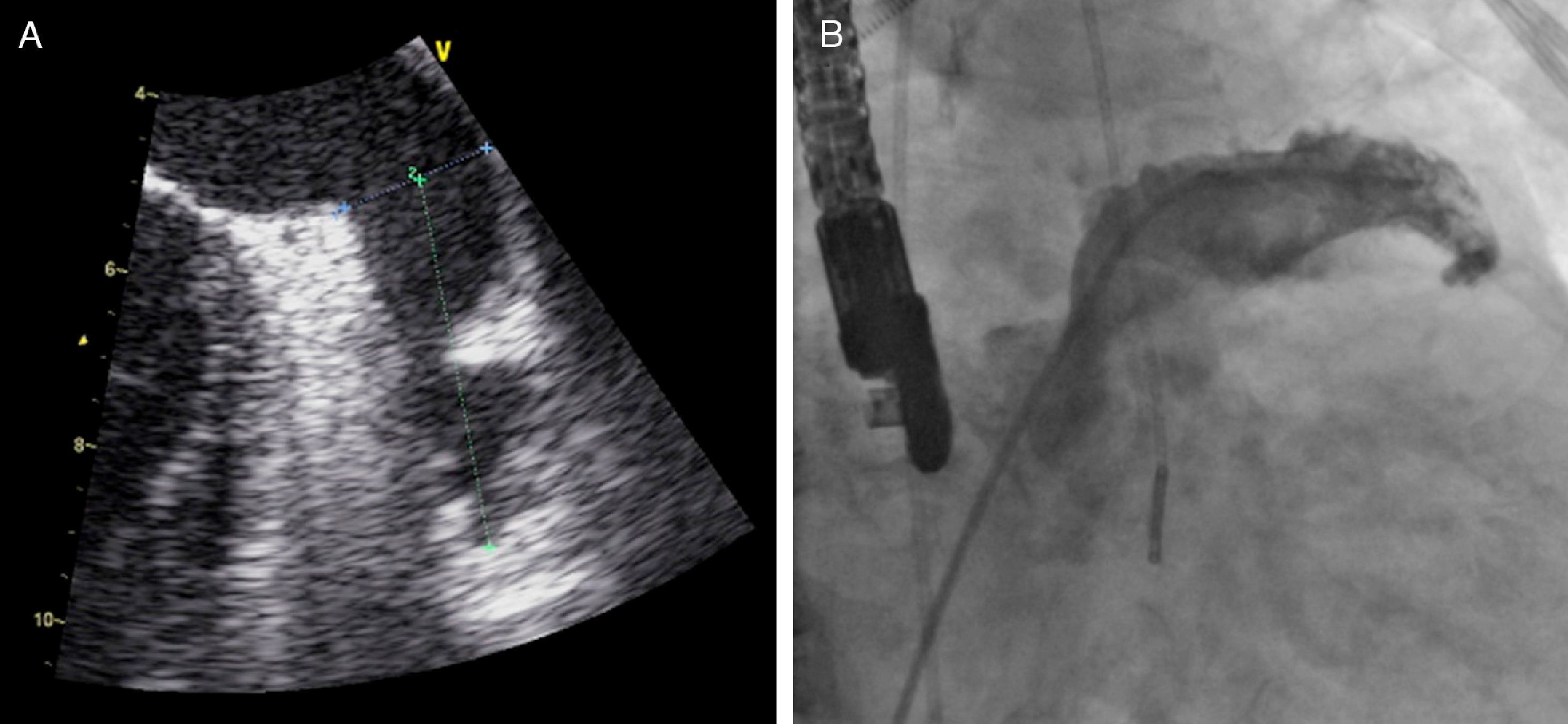
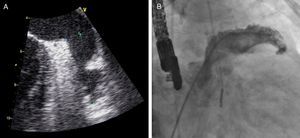
TEE was performed under deep sedation or general anesthesia at 0°, 45°, 90° and 135° to confirm the absence of thrombi in the LAA, and to measure its depth and the widest diameter of its orifice (Figure 3).
Transseptal puncture was guided by fluoroscopy and TEE and a superstiff guidewire was inserted, through which a multipurpose catheter was advanced to the LAA, followed by two contrast injections (right anterior oblique [RAO] 20°, caudal 20° and RAO 20°, cranial 20°), to fill the LAA for measuring purposes.
The ACP was the first device to be used at our center, the Watchman only being used more recently.
The device size was selected according to echocardiographic and angiographic measurements of the LAA in order to ensure that the lobe of the ACP was 2 mm wider than the LAA orifice (requiring an orifice diameter of 12.6–28.5 mm and a landing zone of ≥10 mm) and that the Watchman device was 10–20% wider than the orifice diameter, which had to be between 16.8 and 30.4 mm.
The ACP was prepared by flushing with saline to expel air bubbles from inside the device during its insertion into the delivery catheter.
The device was implanted in the LAA and its position and stability assessed by echocardiography and fluoroscopy. The ACP was considered to be correctly positioned if two-thirds of the lobe was distal to the circumflex artery on TEE and showed a degree of compression of the lobe by the LAA. Successful implantation was defined as absence of significant blush on fluoroscopy and peri-device flow of <3 mm on TEE, in accordance with the classification proposed by Ostermayer et al.26
The position of the Watchman device was assessed using the PASS criteria: position – the device should be distal to or at the LAA orifice; anchor – the stability of the device should be tested under fluoroscopic monitoring: the anchors should be engaged and the device should be stable; if it moves to a site showing a lesser degree of pressure or apposition, it should be repositioned; size – the device should be compressed by 8–20% of its original size, as measured by TEE after positioning; seal – the device should span the orifice so that there is no peri-device flow.
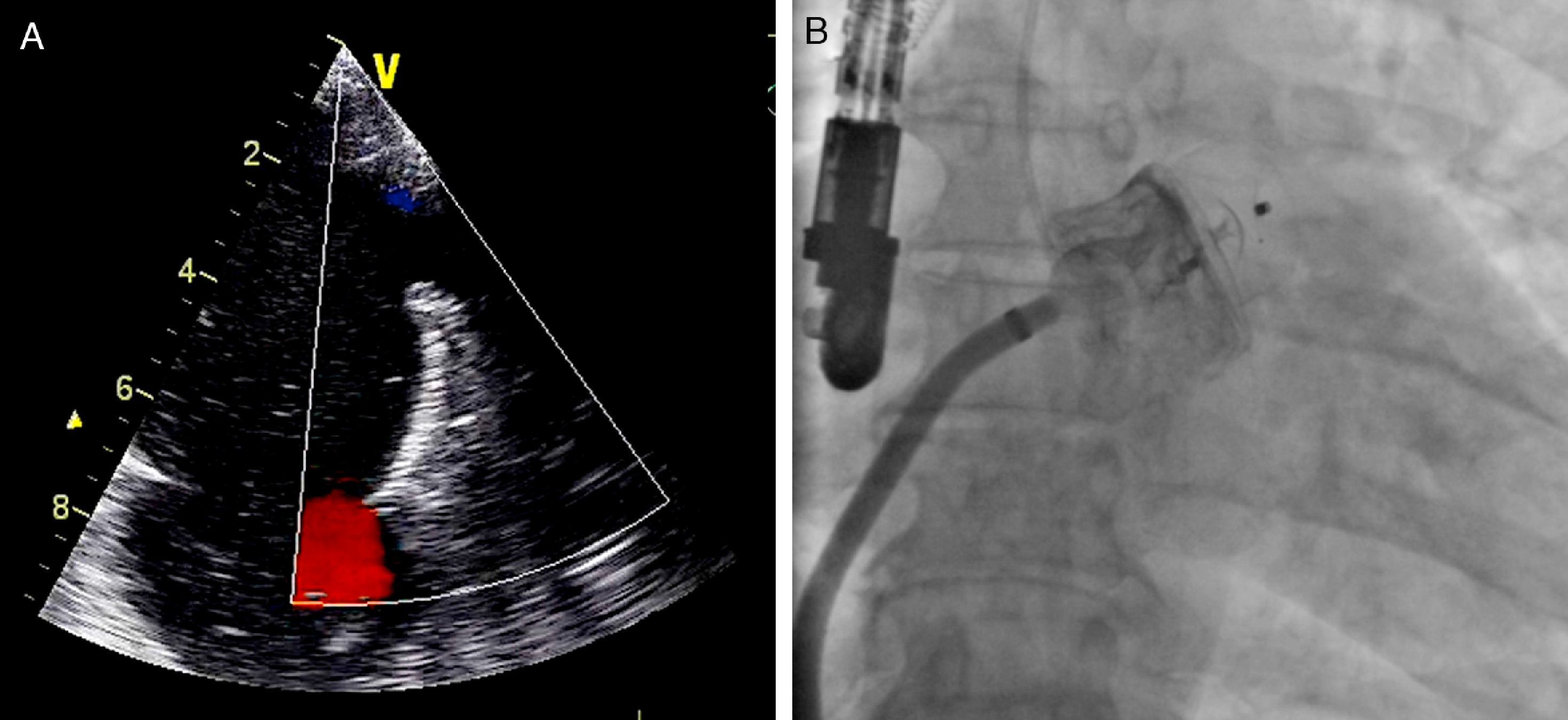
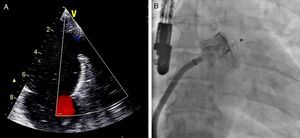
When the positioning criteria were satisfied, the device was released and fluoroscopic and echocardiographic assessment was repeated (Figure 4) to identify possible immediate complications such as device dislodgement or migration, formation of intracardiac or device thrombus, pericardial effusion, compression of the circumflex artery or left superior pulmonary vein, or presence of residual flow. If the criteria were not met, the device was completely or partially recaptured and repositioned before being released.
Following the procedure, patients were medicated with DAT (aspirin 100 mg i.d. and clopidogrel 75 mg i.d.) for one month and single antiplatelet therapy indefinitely. DAT was chosen as a result of the inclusion criteria adopted, since in most patients OAC was contraindicated or impractical. The duration of therapy for each antiplatelet agent was determined on the basis of previous studies on the ACP device19; the Watchman devices were implanted before the results of the ASAP trial25 were known.
Patients remained in hospital under clinical surveillance for up to 24 hours after the procedure to monitor for access-related complications, bleeding, and hemodynamic stability. Transthoracic echocardiography was performed a day after the procedure to rule out pericardial effusion, device dislodgement or migration, peri-device flow and thrombi adhering to the device.
One month after closure patients underwent TEE to assess for signs of incomplete endothelialization (peri-device flow of ≥3 mm), thrombus adhering to the device, device dislodgement or migration, or signs of compression of the circumflex artery or left superior pulmonary vein. If no complications were detected, DAT was replaced by a single antiplatelet; if signs of incomplete endothelialization were detected DAT was continued, while thrombus formation was treated by anticoagulation therapy.
Follow-up was maintained with transthoracic echocardiographic assessment at three, six and nine months and TEE at 12 months to screen once again for local complications.
Clinical monitoring was maintained at the same time as echocardiographic assessment and as required and adverse events were recorded, including death, stroke/TIA, or need for surgery due to periprocedural or device-related complications.
Statistical analysisA descriptive analysis was performed of quantitative variables, expressed as means and standard deviation, and of categorical variables, which were expressed as absolute and relative frequencies.
Relative frequencies are expressed as percentages rounded to one decimal place. The statistical analysis was performed using SPSS version 17.
ResultsPopulation characteristicsPercutaneous LAA closure was successful in 22 of the 23 patients initially selected.
Mean CHADS2 score was 3.2±0.9, mean CHA2DS2-VASC score was 4.7±1.4, and mean HAS-BLED score was 3.7±1.3 (≥3 in 81.8% of cases). AFA was permanent in 72.7%, persistent in 13.6% and paroxysmal in 13.6% of patients.
The characteristics of the study population are presented in Table 1.
Characteristics of the study population.
| Characteristics | |
| Age (years) | 70±9 |
| Male, n (%) | 15 (68.2) |
| Type of AF, n (%) | |
| Paroxysmal | 3 (13.6) |
| Persistent | 3 (13.6) |
| Permanent | 16 (72.7) |
| CHADS2score | 3.2±0.9 |
| CHADS2risk (%/year) | 6.7±2.2 |
| CHA2DS2-VASC score | 4.7±1.4 |
| CHA2DS2-VASC risk (%/year) | 6.2±2.7 |
| HAS-BLED score | 3.7±1.3 |
| HAS-BLED score ≥3, n (%) | 18 (81.8) |
AF: atrial fibrillation; CHADS2 risk: stroke/TIA risk/year expected on the basis of CHADS2 score; CHA2DS2-VASC risk: stroke/TIA risk/year expected on the basis of CHA2DS2-VASC score.
Twelve patients (54.5%) were selected on the basis of contraindication to OAC, due to acquired sideroblastic anemia (n=1; 4.5%), history of cerebral hemorrhage (n=2; 9.1%; one case of spontaneous bleeding under OAC and another following craniocerebral trauma), severe bleeding under warfarin therapy (n=6; 27.3%), and severe bleeding under single antiplatelet therapy (n=3; 13.6%). In eight patients (36.4%) OAC was not an option due to labile INR (n=4; 18.2%) or to difficulties in monitoring INR and high thrombotic and bleeding risk (n=4; 18.2%). Another two patients (9.1%) were selected following failure of OAC therapy, after suffering ischemic stroke with therapeutic INR and detection of LAA thrombus.
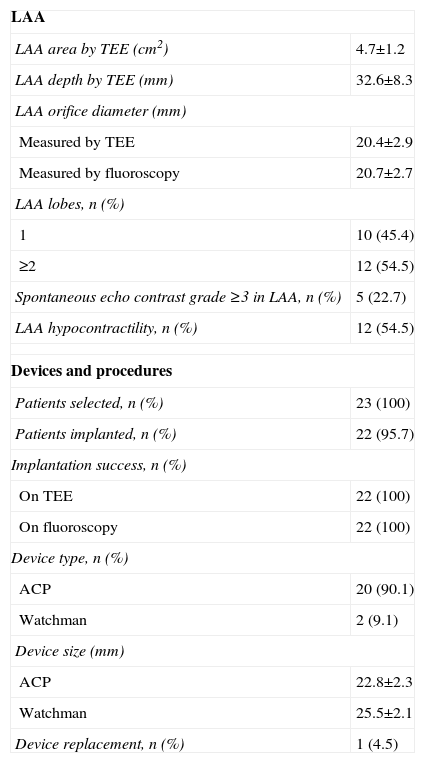
Procedural successLAA closure was unsuccessful in only one of the 23 selected patients, due to the presence of diffuse venous disease that ruled out vascular access. The overall success rate was therefore 95.7%.
The first device selected was successfully implanted in all cases except one (95.4%), in which the device was initially oversized and was replaced, without complications. The final result of all implantations was satisfactory on the basis of the criteria used, as documented by color Doppler TEE and contrast fluoroscopy immediately after implantation.
The ACP device was used in 20 patients (90.9%), while the Watchman device was used in two more recent procedures.
Table 2 shows the characteristics of the LAA, devices and procedures.
Characteristics of the LAA, devices and procedures.
| LAA | |
| LAA area by TEE (cm2) | 4.7±1.2 |
| LAA depth by TEE (mm) | 32.6±8.3 |
| LAA orifice diameter (mm) | |
| Measured by TEE | 20.4±2.9 |
| Measured by fluoroscopy | 20.7±2.7 |
| LAA lobes, n (%) | |
| 1 | 10 (45.4) |
| ≥2 | 12 (54.5) |
| Spontaneous echo contrast grade ≥3 in LAA, n (%) | 5 (22.7) |
| LAA hypocontractility, n (%) | 12 (54.5) |
| Devices and procedures | |
| Patients selected, n (%) | 23 (100) |
| Patients implanted, n (%) | 22 (95.7) |
| Implantation success, n (%) | |
| On TEE | 22 (100) |
| On fluoroscopy | 22 (100) |
| Device type, n (%) | |
| ACP | 20 (90.1) |
| Watchman | 2 (9.1) |
| Device size (mm) | |
| ACP | 22.8±2.3 |
| Watchman | 25.5±2.1 |
| Device replacement, n (%) | 1 (4.5) |
ACP: Amplatzer Cardiac Plug; LAA: left atrial appendage; TEE: transesophageal echocardiography.
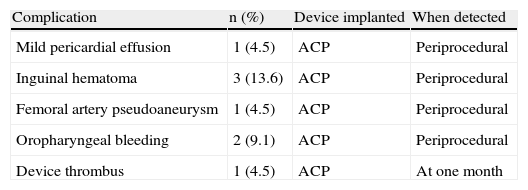
During the first 24 hours after the procedure there were two cases of oropharyngeal bleeding related to intubation, resolved within a few hours by local hemostatic measures, and three puncture site-related inguinal hematomas, one complicated by a femoral artery pseudoaneurysm, all resolved by minimally invasive measures. One case of mild pericardial effusion was also detected on transthoracic echocardiography, which resolved spontaneously. The main complications are summarized in Table 3.
Complications in the periprocedural period and during follow-up.
| Complication | n (%) | Device implanted | When detected |
| Mild pericardial effusion | 1 (4.5) | ACP | Periprocedural |
| Inguinal hematoma | 3 (13.6) | ACP | Periprocedural |
| Femoral artery pseudoaneurysm | 1 (4.5) | ACP | Periprocedural |
| Oropharyngeal bleeding | 2 (9.1) | ACP | Periprocedural |
| Device thrombus | 1 (4.5) | ACP | At one month |
No other periprocedural complications were observed, including device dislodgement or embolization, stroke/TIA, death, need for surgery due to periprocedural or device-related complications, or severe bleeding with need for transfusion.
MedicationAfter the procedure, most patients followed the proposed therapeutic regimen of DAT for one month followed by single antiplatelet therapy indefinitely. This was changed in four patients due to complications. One did not take antiplatelet drugs due to a history of severe bleeding under single antiplatelet therapy as well as under OAC, associated with a major periprocedural vascular complication (femoral artery pseudoaneurysm and significant hematoma at the puncture site). The two patients with history of embolic stroke and documented LAA thrombus under warfarin therapy at therapeutic INR levels were prescribed DAT for one month combined with dabigatran 110 mg 2 i.d., and thereafter continued OAC. In another patient DAT was replaced after one month by subcutaneous enoxaparin 1 mg/kg every 12 hours when a thrombus was detected adhering to the device. Anticoagulation with enoxaparin was continued for five months until the thrombus resolved, at which point single antiplatelet therapy was begun (since this patient had a history of severe bleeding under OAC with therapeutic INR).
Complications during follow-upControl TEE one month after the procedure identified a thrombus on the atrial face of the ACP in one patient; DAT was replaced by enoxaparin 1 mg/kg every 12 hours and stricter echocardiographic control was instituted, with assessments at one week (which documented a slight regression of the thrombus), then monthly up to six months post-procedure, when the thrombus had almost completely resolved. Given the patient's history of severe bleeding under OAC with therapeutic INR, single antiplatelet therapy was begun. Thrombophilia and immunological tests were negative. Three months later the patient presented subacute anemia without evident blood loss; investigation revealed colon cancer, which was treated by surgery and chemotherapy. No thrombus was visible on TEE 12 months after LAA closure.
Only one case of peri-device flow was identified, which according to the classification of Ostermayer et al.26 was mild (color jet width <3 mm), detected on one-month follow-up TEE following implantation of a Watchman device.
During a mean follow-up of 12±8 months, there were no deaths or other complications or thromboembolic events.
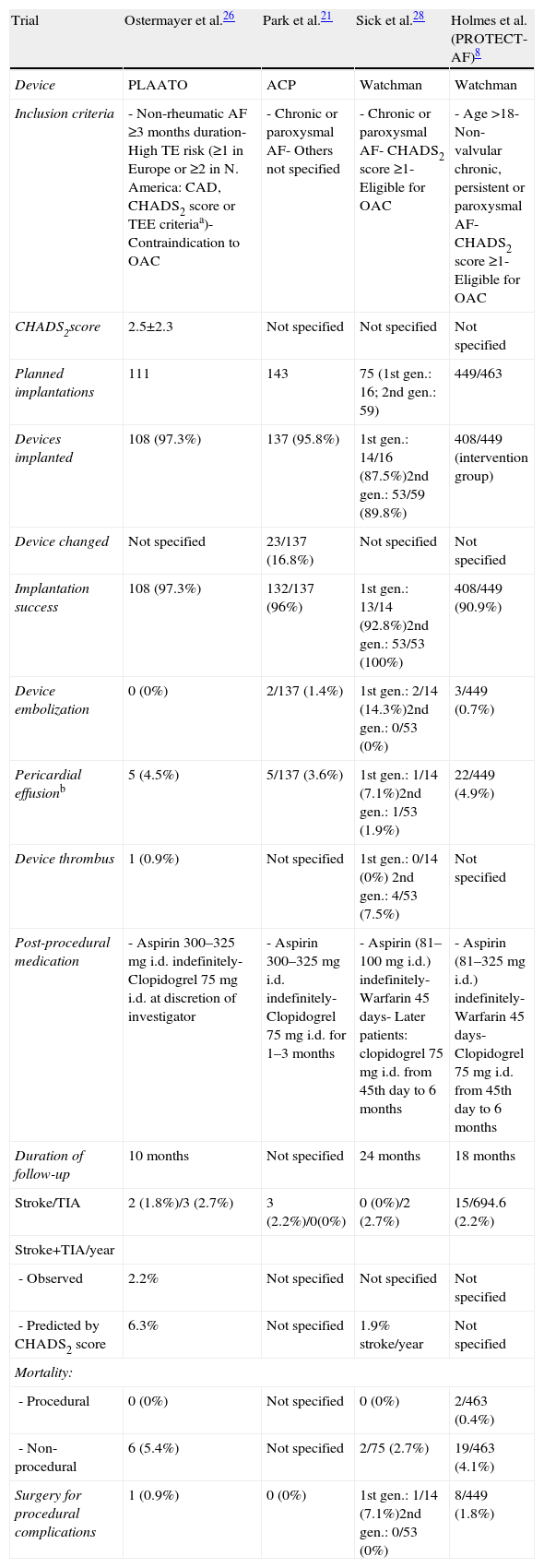
DiscussionThe data presented demonstrate the feasibility of percutaneous LAA closure, which was successful in almost all patients in whom it was attempted; the only failure was due to difficulty in vascular access rather than problems with the implantation technique. Our success rate is similar to those of the main published series on percutaneous LAA closure (Table 4).19
Main results and complications in the principal published series on left atrial appendage closure devices.
| Trial | Ostermayer et al.26 | Park et al.21 | Sick et al.28 | Holmes et al.(PROTECT-AF)8 |
| Device | PLAATO | ACP | Watchman | Watchman |
| Inclusion criteria | - Non-rheumatic AF ≥3 months duration- High TE risk (≥1 in Europe or ≥2 in N. America: CAD, CHADS2 score or TEE criteriaa)- Contraindication to OAC | - Chronic or paroxysmal AF- Others not specified | - Chronic or paroxysmal AF- CHADS2 score ≥1- Eligible for OAC | - Age >18- Non-valvular chronic, persistent or paroxysmal AF- CHADS2 score ≥1- Eligible for OAC |
| CHADS2score | 2.5±2.3 | Not specified | Not specified | Not specified |
| Planned implantations | 111 | 143 | 75 (1st gen.: 16; 2nd gen.: 59) | 449/463 |
| Devices implanted | 108 (97.3%) | 137 (95.8%) | 1st gen.: 14/16 (87.5%)2nd gen.: 53/59 (89.8%) | 408/449 (intervention group) |
| Device changed | Not specified | 23/137 (16.8%) | Not specified | Not specified |
| Implantation success | 108 (97.3%) | 132/137 (96%) | 1st gen.: 13/14 (92.8%)2nd gen.: 53/53 (100%) | 408/449 (90.9%) |
| Device embolization | 0 (0%) | 2/137 (1.4%) | 1st gen.: 2/14 (14.3%)2nd gen.: 0/53 (0%) | 3/449 (0.7%) |
| Pericardial effusionb | 5 (4.5%) | 5/137 (3.6%) | 1st gen.: 1/14 (7.1%)2nd gen.: 1/53 (1.9%) | 22/449 (4.9%) |
| Device thrombus | 1 (0.9%) | Not specified | 1st gen.: 0/14 (0%) 2nd gen.: 4/53 (7.5%) | Not specified |
| Post-procedural medication | - Aspirin 300–325 mg i.d. indefinitely- Clopidogrel 75 mg i.d. at discretion of investigator | - Aspirin 300–325 mg i.d. indefinitely- Clopidogrel 75 mg i.d. for 1–3 months | - Aspirin (81–100 mg i.d.) indefinitely- Warfarin 45 days- Later patients: clopidogrel 75 mg i.d. from 45th day to 6 months | - Aspirin (81–325 mg i.d.) indefinitely- Warfarin 45 days- Clopidogrel 75 mg i.d. from 45th day to 6 months |
| Duration of follow-up | 10 months | Not specified | 24 months | 18 months |
| Stroke/TIA | 2 (1.8%)/3 (2.7%) | 3 (2.2%)/0(0%) | 0 (0%)/2 (2.7%) | 15/694.6 (2.2%) |
| Stroke+TIA/year | ||||
| - Observed | 2.2% | Not specified | Not specified | Not specified |
| - Predicted by CHADS2 score | 6.3% | Not specified | 1.9% stroke/year | Not specified |
| Mortality: | ||||
| - Procedural | 0 (0%) | Not specified | 0 (0%) | 2/463 (0.4%) |
| - Non-procedural | 6 (5.4%) | Not specified | 2/75 (2.7%) | 19/463 (4.1%) |
| Surgery for procedural complications | 1 (0.9%) | 0 (0%) | 1st gen.: 1/14 (7.1%)2nd gen.: 0/53 (0%) | 8/449 (1.8%) |
a TTE criteria – flow velocity in the LAA <20 cm/s or moderate or dense spontaneous echocardiographic contrast. b Requiring treatment (pericardiocentesis or surgery). 1st gen.: first-generation device; 2nd gen.: second-generation device; ACP: Amplatzer Cardiac Plug; AF: atrial fibrillation; OAC: oral anticoagulation; TEE: transesophageal echocardiography; TIA: transient ischemic attack.
Our recent experience with the Watchman shows that the availability of a second device will bring advantages in terms of a wider range of sizes and different conformations and technical aspects, making it easier to select the device that is best adapted to each patient's anatomy.
With regard to the safety of the technique, the only complications observed in our series were mild and resolved either spontaneously or after minimally invasive measures and without functional repercussions.
The most commonly reported complication in percutaneous LAA closure is severe pericardial effusion with hemodynamic compromise requiring pericardiocentesis, but this was not seen in our series; nor were other reported complications such as device dislodgement or migration, need for surgery, or procedure-related death (Table 4).19
The most serious complication in our series was device thrombus, which may have been due to a subsequently diagnosed paraneoplastic syndrome. There is no agreement concerning the best antithrombotic protocol to adopt in these patients, since OAC was contraindicated or impractical in most of them, unlike the population of the PROTECT AF trial, all of whom underwent warfarin therapy for 45 days after LAA closure. However, we consider that our treatment protocol and follow-up were appropriate, since the thrombotic complication mentioned above was identified rapidly, enabling treatment to be adjusted accordingly.
Although the patients in this study had high bleeding risk, with a mean HAS-BLED of 3.7±1.3 (≥3 in 81.8% of patients), no severe bleeding occurred, either periprocedurally or during follow-up. We therefore consider that the antiplatelet therapy implemented was adequate.
Most of the complications observed occurred in patients implanted with the ACP device, but this was to expected given that this was used in 90.1% of cases.
The only case of peri-device flow was mild according to Ostermayer et al.’s classification.26 A recent study by Viles-Gonzalez et al.,27 based on a retrospective analysis of the intervention group in PROTECT AF, showed that the incidence of peri-device flow does not increase significantly over time, nor does it increase thromboembolic risk or significantly alter prognosis. These findings were independent of severity of flow and of duration of warfarin therapy.27
The series presented included patients with higher thromboembolic risk than in the main published series,19 with a higher mean CHADS2 score (3.2±0.9), due to the selection criteria used. The stroke/TIA rate observed (0% in 12±8 months) was lower than that expected on the basis of the CHADS2 score (6.8±2.2%/year) and CHA2DS2-VASC score (6.4±2.5%/year), as seen in various published series.19
ConclusionsIn patients with AF and high thromboembolic and bleeding risk, percutaneous LAA closure was feasible, safe and effective, with a lower stroke/TIA rate than that expected on the basis of the CHADS2 and CHA2DS2-VASC scores. This non-pharmacological treatment can therefore be considered an alternative for patients in whom OAC is impractical, contraindicated or ineffective.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Please cite this article as: Faustino, A, et al. Encerramento percutâneo do apêndice auricular esquerdo paraprofilaxia de tromboembolismo na fibrilhação auricular em doentes com contraindicação ou falência da hipocoagulação oral: experiência de um serviço. Rev Port Cardiol 2013. http://dx.doi.org/10.1016/j.repc.2012.10.011.